Journal of Fisheries science and Technology - No.3/2016
ABSTRACT There has recently been an increasing demand to produce protein hydrolysates containing peptides with specifi c biological properties, which could be marketed as functional food ingredients. The objective of this study was to evaluate the in vitro angiotensin converting enzyme inhibitory activity of striped catfi sh skin hydrolysates and its corresponding fractionates. The striped catfi sh skin from fi llet processing was extracted in an autoclave at 1210C for 30 minutes to obtain an extracted protein. Then it was further hydrolysed with Alcalase with the enzyme to substrate ratio of 20 units/gram protein at 50oC, pH 8 for 7h to obtain protein hydrolysate. The degree of hydrolysis (DH) increased with the increase of hydrolysis time and reached the highest DH of 91.9% after 7h hydrolysis. The 5-H hydrolysate (DH= 60.8%) exhibited the highest ACE-inhibitory activity (IC50 = 831 µg/ml). Therefore, the 5-h hydrolysate sample was used as material for studying enrichment of ACE-inhibitory peptides by ultrafi ltration using three different molecular weight cut-off membranes (10, 5, and 1 kDa). Six sample fractions obtained during ultrafi ltration process (permeate and retentate) were tested for angiotensin converting enzyme inhibition activity. Permeate of 1 kDa membrane showed the highest activity. The obtained hydrolysates were fractioned using SephadexM G-15. Based on gel fi ltration chromatography results, angiotensin converting enzyme inhibitory peptides had molecular weight ranging of 307 Da to 429 Da. Our fi ndings revealed the potential of using catfi sh skin as a promising material for retrieving angiotensin converting enzyme inhibitory substances

Trang 1

Trang 2

Trang 3
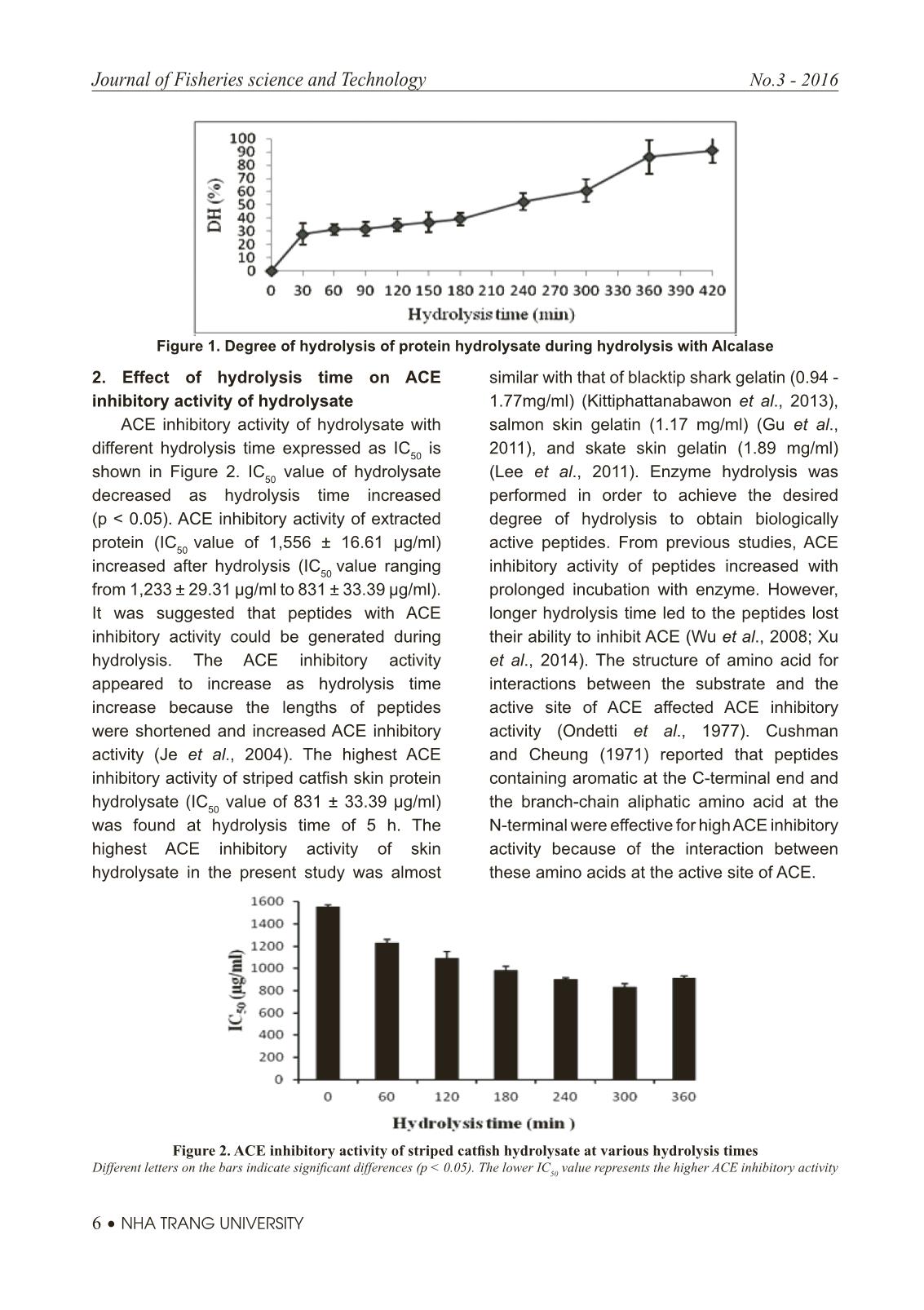
Trang 4
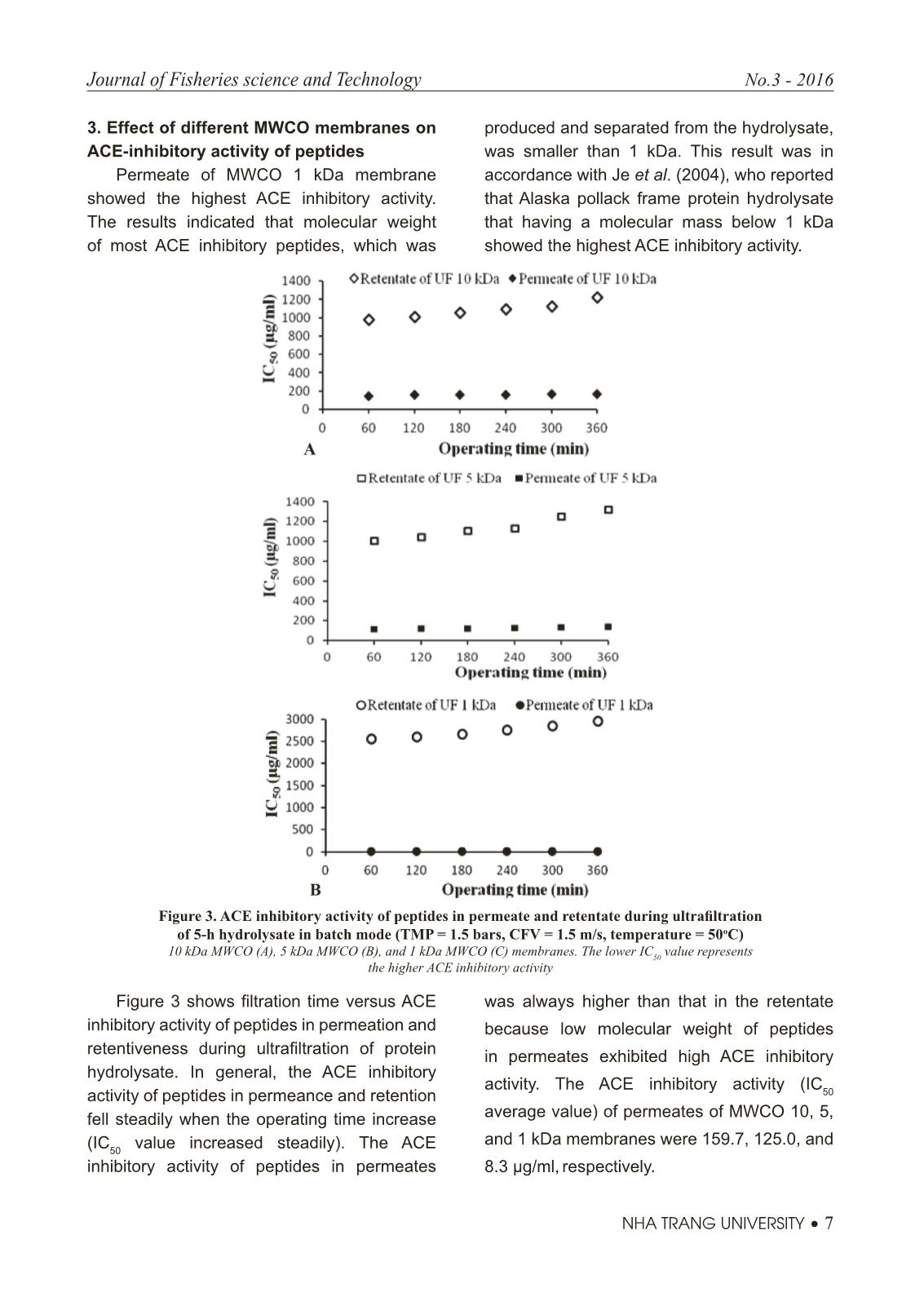
Trang 5

Trang 6

Trang 7

Trang 8

Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Journal of Fisheries science and Technology - No.3/2016

distributions and cohort slicing analysis, the data were used to compute for only males. The total length was calculated based on the relationship equation with CL and then the asymptotic length (L∞) was estimated based on total length according to Sarda et al., (1998) [13]. The length-frequency distributions were plotted by year and analysed as a combination of cohort length distributions, each of which was assumed to be in the form of a Gaussian distribution [15]. The mean length at age of each cohort and the standard deviations was estimated and then the average growth rate can be modelled. The von-Bertalanffy (VB) growth equation was applied to the estimated mean length-at-age and the standard deviation data according to the equation: Lt = L∞ (1− e −k(t−t0)) (where Lt as the average length-at-age t, L∞ as the asymptotic length, k as the growth rate parameter and t0 as hypothetical age at length equal to 0). The VB growth model assumes that growth was faster when the species was young and get slow onwards after they become mature. In order to run this equation in R, some initial values as L∞, k and t0 were set and the least sums of squared error (SSE) was minimised using an iterative process [15]. The parameter t0 was fi xed at 0.1 as the fi rst two age groups were completely missing from the data series and t0 was always estimated really low. To compare the estimated growth parameters of nephrops in this study with other studies, the growth performance index (Φ’) were used which calculated according to equation: Φ = log10k + 2 * log10 L∞ (where k as the growth rate parameter and L∞ as the asymptotic length). These data were analysed by models in the R software package according to Stefansson (2012) [14]. III. RESULTS AND DISCUSSION 1. Characteristics of age distribution 1.1. Age distribution at different years The age distribution of lobster population from 1970 to 2011 had twenty fi ve age groups corresponding several peaks of carapace length (CL) frequency distribution (Figure 1). The mean CL of N. norvegicus at age estimated was ranged from 7.22 mm at age 1 to 76.08 mm at age 25. 124 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No.3 - 2016 Figure 1. The age distribution of N. norvegicus based on CL distribution in catches from 1970 to 2011 The growth rate of nephrops was high in the fi rst four years with mean increment of about 7.0 mm per year. After this age, they reached a maturity size, therefore, the growth rate gradually decreased. The proportions corresponding to relative ages revealed a difference between decades. In the period 1970 - 1979, the age groups of 5 to 9 year olds were in high abundant as the mean CL ranged 32.41 - 49.19 mm. The age groups of 5 to 10 year olds of Nephrops showed a dominant proportion in the catches in the periods from 1980 - 1989 corresponding to mean CL of 32.41 - 52.42 mm. On the other hand, in recent years (1990 - 2009) and the whole data from 1970 to 2011, the landings showed high abundant of age groups from 6 to 10 year olds or mean CL of 37.26 - 52.42 mm. For the other groups, the age proportions were small (Table 1). Table 1. Mean CL and age proportion of N. norvegicus in catches from 1970 to 2011 Age Mean CL (mm) Proportion (%) SD 1970-1979 1980-1989 1990-1999 2000-2009 1970-2011 1 7.22 3.904e-04 4.026e-04 9.454e-05 3.805e-06 1.471e-04 0.37146 2 14.51 6.690e-04 7.699e-04 1.606e-04 1.172e-04 2.719e-04 0.74646 3 21.09 0.00333 0.00135 6.967e-04 4.670e-04 0.00155 1.08544 4 27.03 0.04052 0.02019 0.02611 0.00776 0.02083 1.39185 5 32.41 0.12999 0.10445 0.09711 0.06046 0.09547 1.66882 6 37.26 0.23606 0.14914 0.20350 0.12269 0.16539 1.91919 7 41.64 0.15564 0.21745 0.19869 0.15398 0.18045 2.14551 8 45.61 0.16582 0.16544 0.14899 0.17378 0.15539 2.35008 9 49.19 0.10511 0.11656 0.11814 0.11922 0.11871 2.35008 10 52.42 0.04347 0.12218 0.10038 0.13062 0.10176 2.70215 11 55.34 0.06881 0.04140 0.06161 0.06519 0.05339 2.85326 12 57.98 0.02776 0.03125 0.03545 0.08298 0.06087 2.98984 13 60.36 6.564e-07 0.01685 1.367e-06 3.115e-06 8.279e-08 3.11330 14 62.52 0.01122 4.244e-07 0.02809 0.05616 0.03708 3.22490 15 64.46 0.00688 0.00959 2.685e-07 0.02014 1.075e-04 3.32578 16 66.22 5.898e-07 3.531e-07 4.401e-07 2.007e-06 9.292e-07 3.41697 17 67.81 2.331e-07 8.992e-08 7.086e-10 6.164e-06 0.00543 3.49939 18 69.25 5.413e-04 5.351e-08 4.058e-07 6.843e-04 0.00211 3.57391 19 70.54 9.469e-04 7.151e-08 1.810e-07 0.00549 2.967e-07 3.64126 20 71.71 3.102e-06 3.660e-08 9.089e-05 2.262e-04 3.539e-07 3.70214 21 72.77 3.665e-07 1.911e-08 2.404e-04 3.802e-06 2.488e-07 3.75713 22 73.73 3.959e-07 8.855e-08 1.732e-04 2.856e-06 7.566e-07 3.80691 23 74.59 6.670e-08 1.921e-07 3.134e-05 1.450e-06 1.331e-07 3.85189 24 75.37 9.341e-08 3.232e-07 5.930e-06 1.744e-06 1.397e-06 3.89253 25 76.08 0.00285 0.00296 4.472e-04 1.065e-06 0.00103 3.92928 NHA TRANG UNIVERSITY • 125 Journal of Fisheries science and Technology No.3 - 2016 The mean CL of this species caught in 1970 was 45.10 mm and presented a fl uctuation onwards with minimum value of 42.60 mm in 1973. Until 1995, the CL increased rapidly and reached a peak of 49.66 mm in 2010. 1.2. Age distribution at different fi shing areas The age distributions of N. norvegicus were compared the Southeast with Southwest fi shing grounds based on the mean of CL frequency distributions. In general, the mean CL of N. norvegicus in catches increased from 1970 to 2011 (Figure 2). Figure 2. The mean CL of N. norvegicus in catches from 1970 to 2011 Figure 3. The age distribution of N. norvegicus based on CL distribution in catches from 1970 to 2011 at different fi shing areas (A: Southeast area and B: the Southwest area). Figure 3 showed that the plotted of age distributions as CL frequency distributions were similar, but in the Southwest areas, the distribution was wider than in the Southeast areas, which revealed a difference in the proportion of age groups (Table 2). 126 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No.3 - 2016 Figure 4 showed that the mean CL of nephrops caught in the Southeast areas fl uctuated but did not show any consistent trend with the mean CL was 44.84 mm from 1970 to 2011. Meanwhile, in the Southwest areas, the mean CL of this species revealed an increase from a minimum value of 40.27 mm in 1970 to maximum value of 61.42 mm in 2003 and then gradually decreased to 50.0 mm in 2011. The mean carapace length of N. norvegicus in this fi shing areas was 47.94 which is signifi cantly higher when compared to Southeast areas (p<0.01). The signifi cant difference of mean CL of N. norvegicus caught in Southwest and Southeast fi shing areas may be caused by different type of bottom structure in their habitat. This was in good agreement with Figure 4. The mean CL of N. norvegicus in catches at different fi shing areas from 1970 to 2011 Table 2. Age proportion of N. norvegicus in catches at different fi shing areas from 1970 to 2010 Age Proportion (%) Age Proportion (%) Southeast areas Southwest areas Southeast areas Southwest areas 1 2.106e-04 2.023e-04 14 0.01436 0.06853 2 3.733e-04 3.489e-04 15 0.00406 0.01124 3 0.00159 0.00144 16 4.538e-04 2.992e-05 4 0.02245 0.02029 17 7.999e-04 0.01403 5 0.11301 0.08017 18 8.431e-04 0.01562 6 0.19184 0.13409 19 6.784e-06 5.318e-08 7 0.19557 0.15062 20 3.183e-07 3.339e-07 8 0.16140 0.13479 21 1.625e-07 2.269e-07 9 0.10207 0.12198 22 1.087e-07 4.741e-07 10 0.10534 0.09338 23 4.469e-07 1.386e-09 11 0.02944 0.07735 24 1.988e-07 7.967e-06 12 0.05462 0.07474 25 0.00155 0.00142 13 5.323e-07 3.542e-07 In the Southeast fi shing areas, age groups with the highest proportions ranged between 5 and 10 corresponding to mean CL of 32.41 - 52.42 mm, while the proportion of age groups 5 to 12 with mean CL ranged from 32.44 mm to 57.98 mm were most abundant in the Southwest fi shing areas. NHA TRANG UNIVERSITY • 127 Journal of Fisheries science and Technology No.3 - 2016 previously observation by Tuck et al. (1997), who stated that local variations in size composition, density and growth of this species might be related to type of bottom sediment [16]. Moreover, the mean CL size of nephrops was positively correlated with increasing clay/silt content from 30 - 90% and showed an inversely relationship in the Irish Sea with bottom sediment ranges between 4 - 49% of clay/silt [16]. Moreover, the variation in mean CL of nephrops is also negatively correlated with depth [5]. In the Southwest areas, the depth ranged from 130 - 180 m and it could explain the higher average CL than found in the Southeast areas with depth of 180 - 250 m. 2. The growth parameters The mean CL at ages of N. norvegicus obtained from the length frequency distribution and cohort slicing analysis were then fi tted to the von Bertalanffy growth curve model to calculate the growth parameters: CL∞ = 82.5 (mm), k = 0.1 (yr -1), t0 = - 0.1 and the growth performance index Φ’) were 3.87. Mytilineou and Sarda (1995), the CL∞ of lobster in the Catalan Sea ranged from 84.4 to 96.6 mm [9]. Meanwhile, the asymptotic length (L∞) calculated based on the relationship equation between CL and total length in this study was 300 mm which was higher than L∞ of 226 mm in Ancona Sea [13]. The growth rate parameter (k) of this species obtained in this study (0.1 yr-1) was higher than those stated by Mytilineou and Sarda (1995) in Catalan Sea (0.05-0.08 yr-1) but consistent with value of 0.11 in Ancona Sea [7]. On the other hand, the growth performance index (Φ,) calculated 3.87 in this study was higher than the results reported by Mytilineou and Sarda (1995) in Catalan Sea (2.6 – 2.8) [9] but lower than values of 6.33 – 6.64 in Ancona Sea [7]. The values of k and Φ’ (0.1 yr-1 and 3.87) obtained in this study were in good agreement with observation before in Iceland (0.1 yr-1 and 2.81, respectively) [4]. These results were contrary to Pauly (1984), who stated that growth performance index was constant [11]. However, it was consistent with other studies on N. norvegicus which presented the growth rate can vary considerably due to environmental forces such as temperature, sediment particle size, food availability, population density and fi shing pressure, each of which may have different and possibly interactive effects [1]. IV. CONCLUSION Age distributions of N. norvegicus in Iceland revealed twenty fi ve age groups corresponding to mean CL ranging from 7.22 mm at age 1 to 76.08 mm at age 25. The dominant age groups in the catch were 5 to 10 year olds. The mean CL of this species increased from 45.10 mm in 1970 to 49.3 mm in 2011. The mean CL of N. norvegicus caught in the Southwest areas Figure 5. von-Bertalanfy growth curve of N. norvegicus at different ages in catches from 1970 to 2011 (Broken lines showed standard deviation) Figure 5 showed that N. norvegicus caught in Icelandic waters had a CL∞ of 82.5 mm that was fairly similar when compared with previous studies in different fi shing areas although using different methods. According to 128 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No.3 - 2016 (47.94 mm) was signifi cantly higher than those of 44.84 mm caught in the Southeast areas (p<0.01). The growth parameters of nephrops were: CL∞ = 82.5 (mm), k = 0.1 (yr -1), t0 = - 0.1 and the growth performance index (Φ’) were 3.87. ACKNOWLEDGEMENTS The authors would like to thank the United Nations University - Fisheries Training Programme and Marine Research Institute in Iceland for great supportted and excellent guidance for this research. REFERENCES 1. Bell M., Redant F., and Tuck I., 2006. Nephrops species. In B. Philipps, Lobsters: Biology, Management, Aquaculture and Fisheries. Oxford: Blackwell Publishing Ltd.: 412-450. 2. Bennett D., 1980. Perspectives on European lobster management. In J. Cobb, & B. Phillips, The Biology and Management of Lobsters (Vol. 2, p. 390). New York: Academic Press. 3. Campbell N., Allan L., Weetman A. and Dobby H., 2009. Investigating the link between Nephrops norvegicus burrow density and sediment coposition in Scottish waters. ICES Journal of Marine Science, 66, 2052-2059. 4. Eiriksson H., 1982. Estimating the growth of Nephrops at Iceland. ICES. Shellfi sh Committee, C.M. 1982/K:16, 9. 5. Eiriksson H., 1999. Spatial variabilities of CPUE and mean size as possible criteria for unit stock demarcations in analytical assessments of Nephrops at Iceland. Rit Fiskideildar 16, 239-245. 6. FAO, 2012. Fisheries and Aquaculture Department. Retrieved December 15, 2012, from Food and Agriculture Organization of the United Nations: fi shery/species/2674/en 7. Marano G., Marsan R., Pastorelli A., Vaccarella R., 1998. Areale di distribuzione e pesca dello scampo, Nephrops norvegicus (L), nelle acque del basso Adriatico. Biology Marine Mediterranean, 2, 284-292. 8. Morello E., Froglia C., Atkinson R., 2007. Underwater television as a fi shery-independent method for stock aassessment of Norway lobster (Nephrops norvegicus) in the Central Adriatic Sea (Italy). ICES Journal of Marine Science, 64, 1116-1123. 9. Mytilineou C. and Sarda F., 1995. Age and growth of Nephrops norvegicus in the Catalan Sea, using length-frequency analysis. Fisheries Research, 23, 283-299. 10. Pampoulie C., Skirnisdottir S., Hauksdottir S., Olafsson K., Eiriksson H., Chosson V., 2011. A pilot genetic study reveals the absence of spatial genetic structure in Norway lobster (Nephrops norvegicus) on fi shing grounds in Icelandic waters. ICES Journal of Marine Science, 68, 20-25. 11. Pauly D., 1984. Some simple methods for the assessment of tropical fi sh stocks. Fish. Tech. Pap. No. 234, p. 52. Rome: FAO. 12. Robertson J. and Shanks A., 1989. Further studies of the size selection of nephrops by different condents. Scottish Fisheries Working. 13. Sarda F., Leonart J. and Cartes J., 1998. An analysis of the population dynamics of Nephrops norvegicus (L.) in the Mediterranean Sea. Scientia Marina, 62, 135-143. 14. Stefansson G., 2012. Yield per recruit analysis. Retrieved January 15, 2012, from Tutor web: net/fi sh510.5 15. Stefansson G. and Taylor L., 2012. Modelling length at age distribution. Retrieved December 24, 2012, from Tutor web: fi sh510.3 16. 16. Tuck I., Chapman C. and Atkinson R., 1997. Population biology of the Norway lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland - I: Growth and density. ICES Journal of Marine Science, 54, 125-135. 17. 17. Tuck I. and Bailey N., 2000. Assessment of Nephrops stocks in Loch Torridon area. Aberdeen: Marine Laboratory Report.
File đính kèm:
 journal_of_fisheries_science_and_technology_no_32016.pdf
journal_of_fisheries_science_and_technology_no_32016.pdf

