Multi-correlation between nematode communities and environmental variables in mangrove-shrimp ponds, Ca Mau province, Southern Vietnam
Multi-correlation between bio-indices of nematode communities and ecological parameters in
mangrove-shrimp farming ponds in Tam Giang commune, Nam Can District, Ca Mau Province,
Vietnam were investigated. In which, diversities of nematode communities and several
environmental variables in eight ponds were considered to process. Our findings underlined the
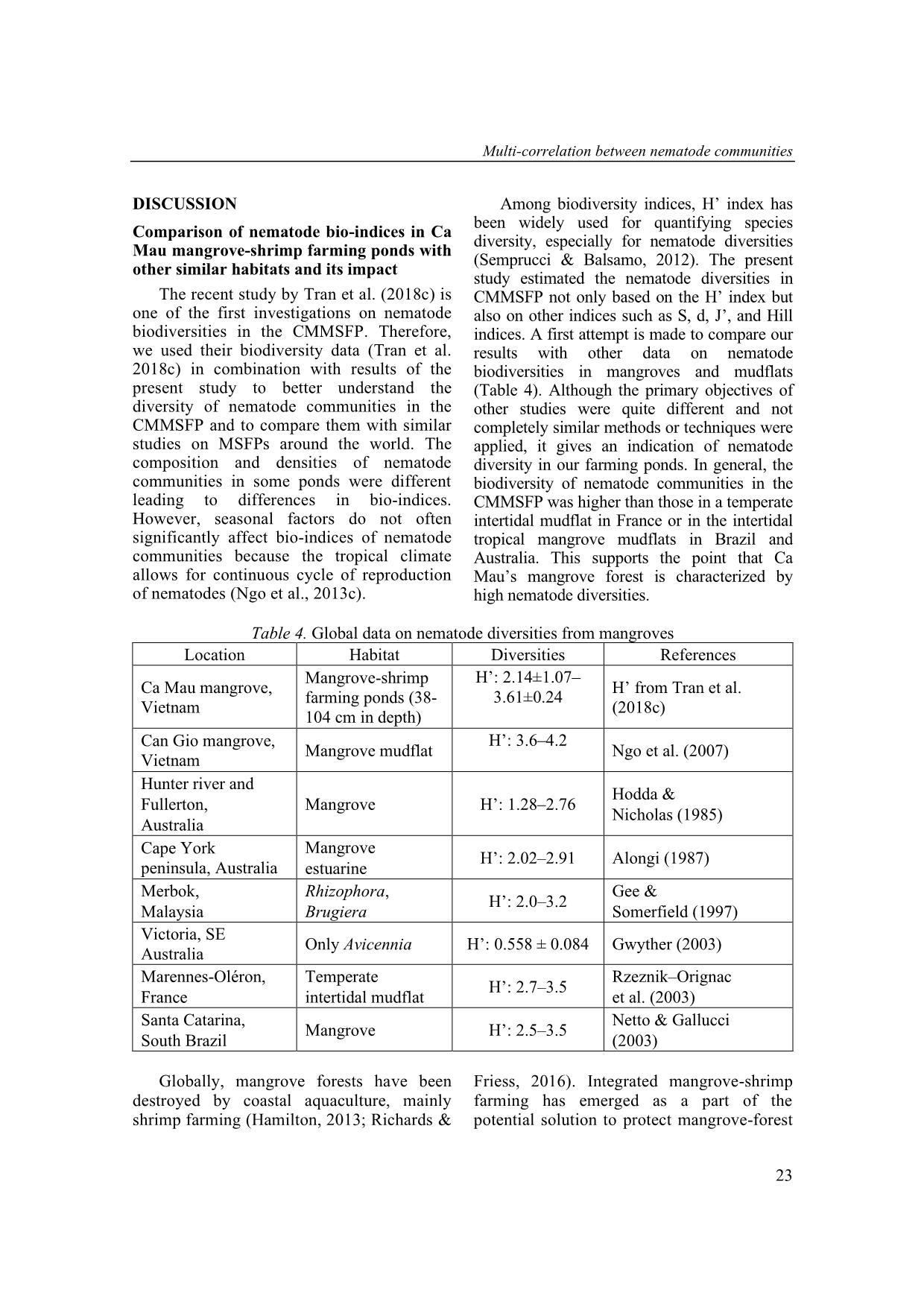
high diversity of nematode communities in mangrove-shrimp farming ponds compared to other
mangrove habitats. Nematode diversities provided more oppotunity in natural food for shrimp.
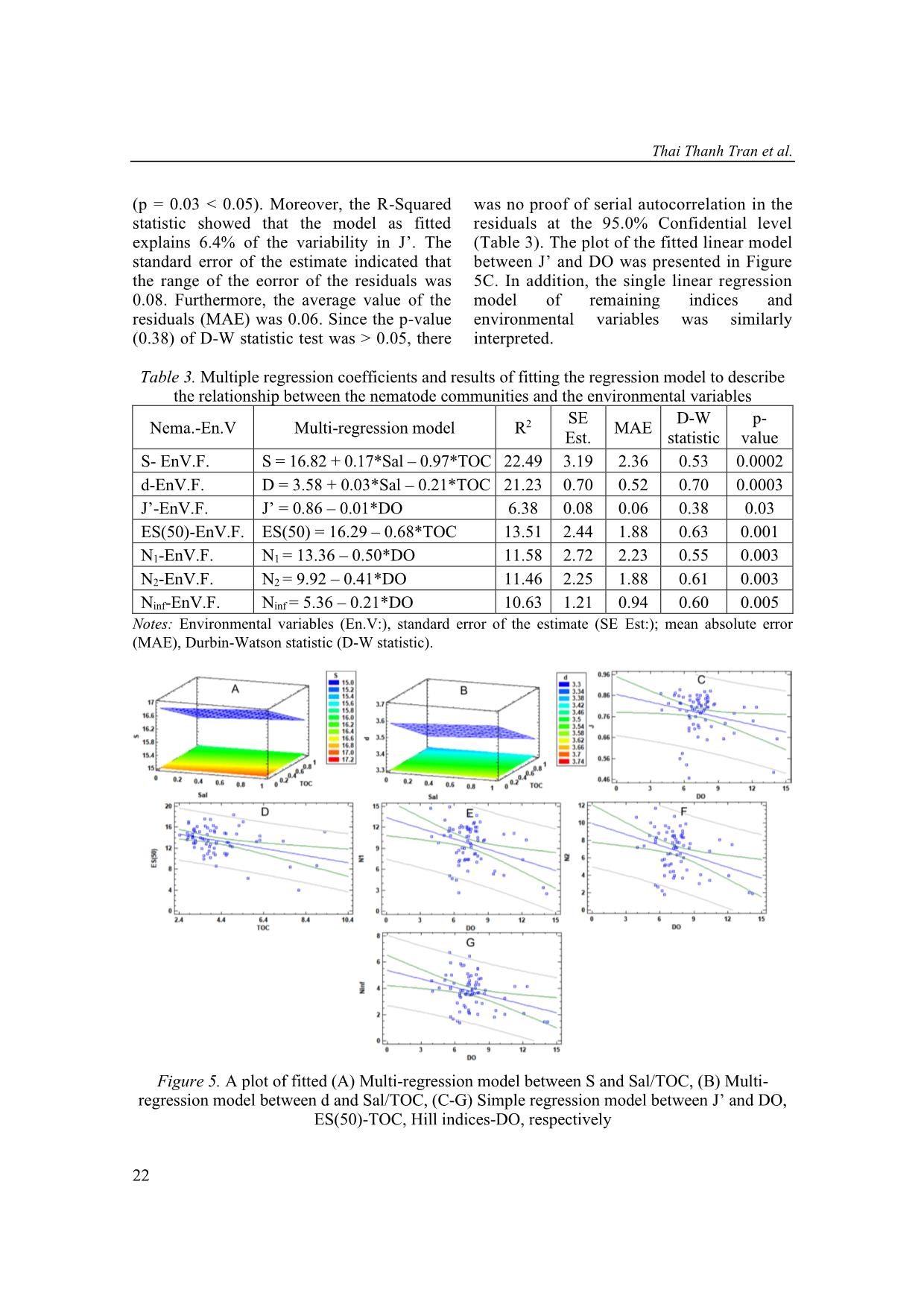
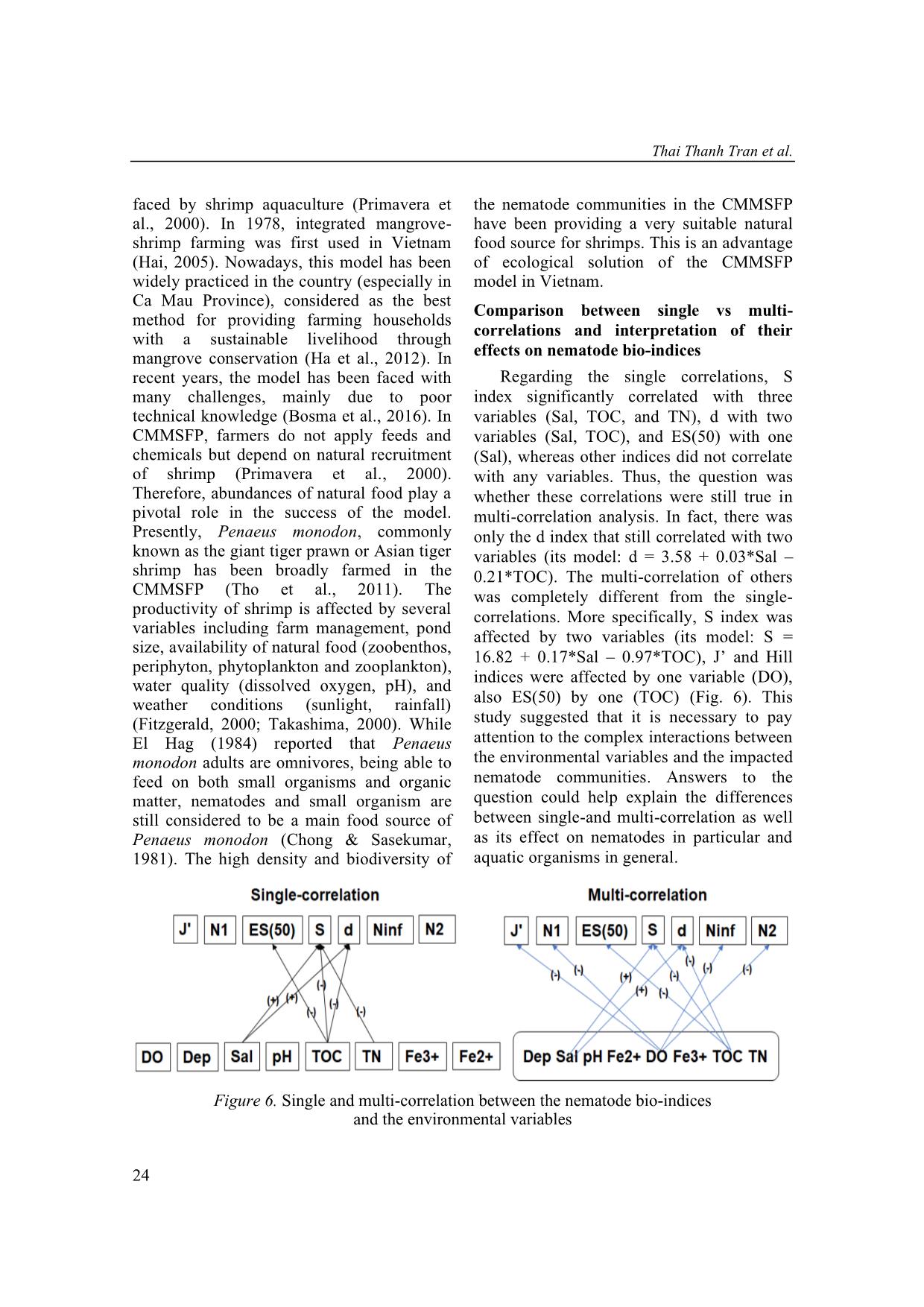
Single correlation analyses showed that the species richness index correlated significantly to
three variables (salinity, total organic carbon, and total nitrogen), the Margalef diversity index
correlated to two variables (salinity, total organic carbon), and the expected number of species
for 50 individuals index correlated with one variable (salinity). Results of multi-correlation
analyses between the nematode bio-indices and the environmental variables were completely
different from those of single-correlation analyses. In multi-correlation analyses, the species
richness and the Margalef diversity index correlated to two variables (salinity, total organic
carbon), Pielou’s evenness index and Hill indices correlated with dissolved oxygen, also the
Hurlbert index correlated to total organic carbon. Hence, it is necessary to pay attention to the
impact of complex interactions between the multi-environmental variables and nematode
communities. This research aims to explain the differences between single- and multi-correlation
for evaluation of the effects of environmental factors on nematodes as well as aquatic organisms.

Trang 1

Trang 2
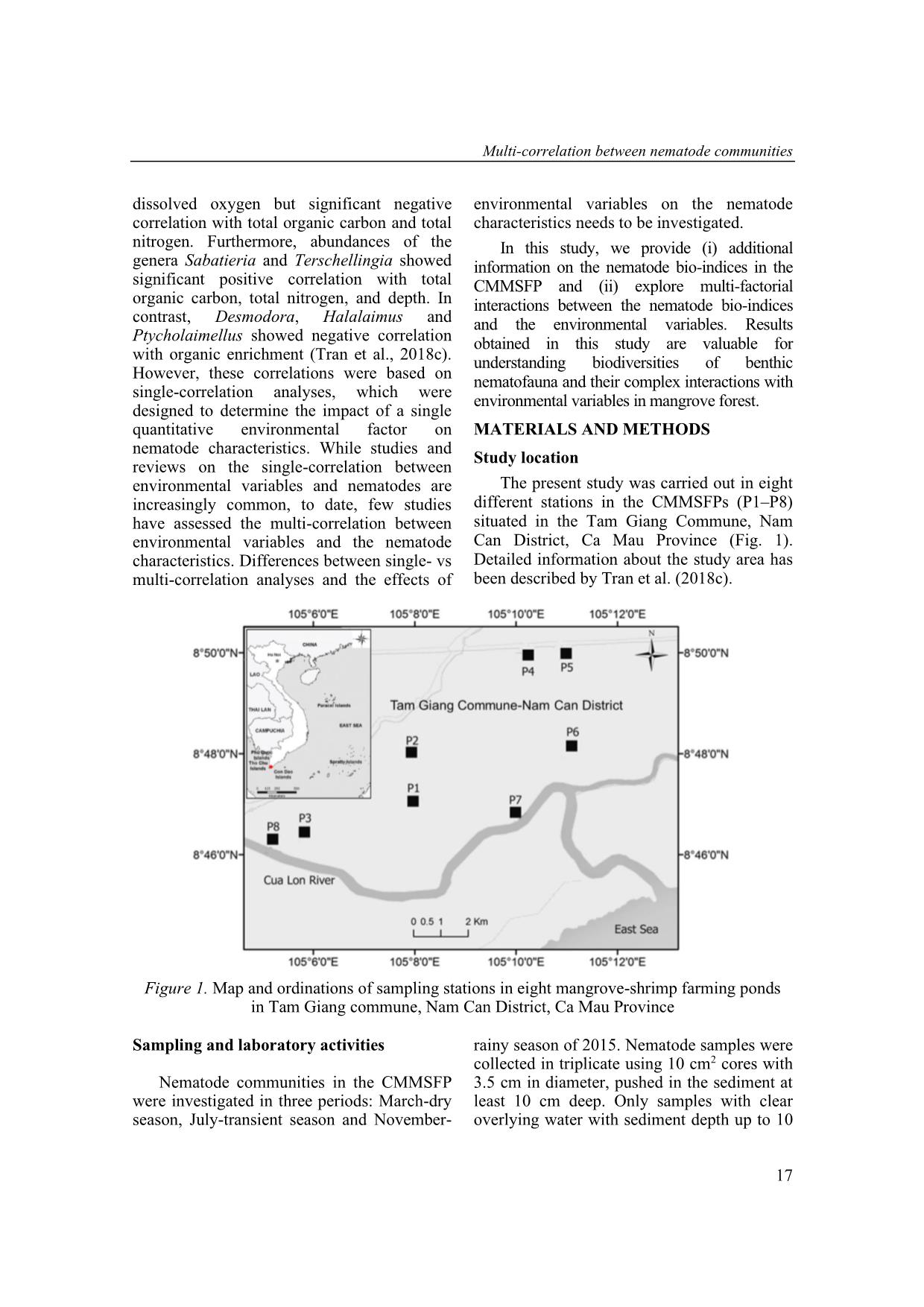
Trang 3

Trang 4
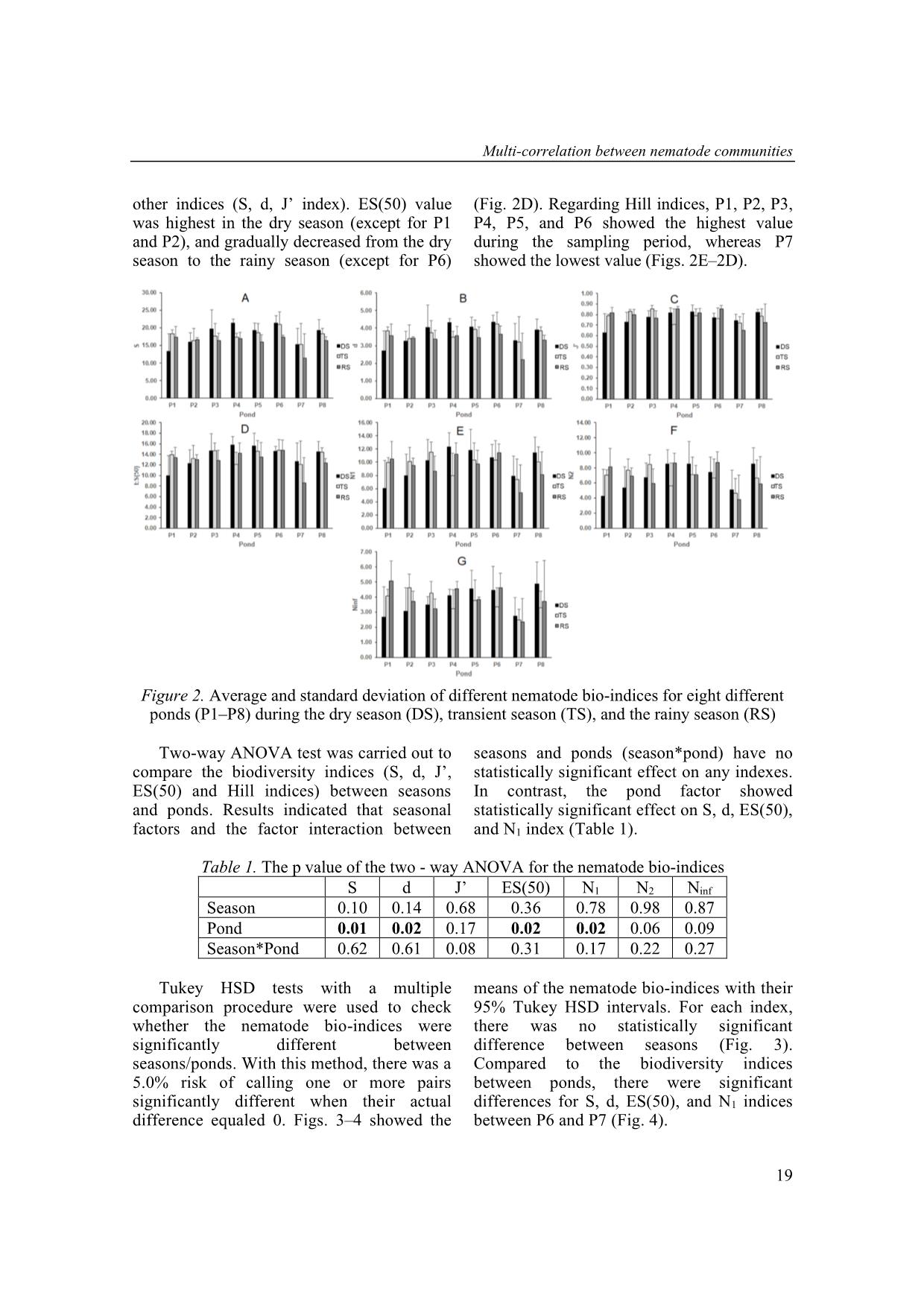
Trang 5
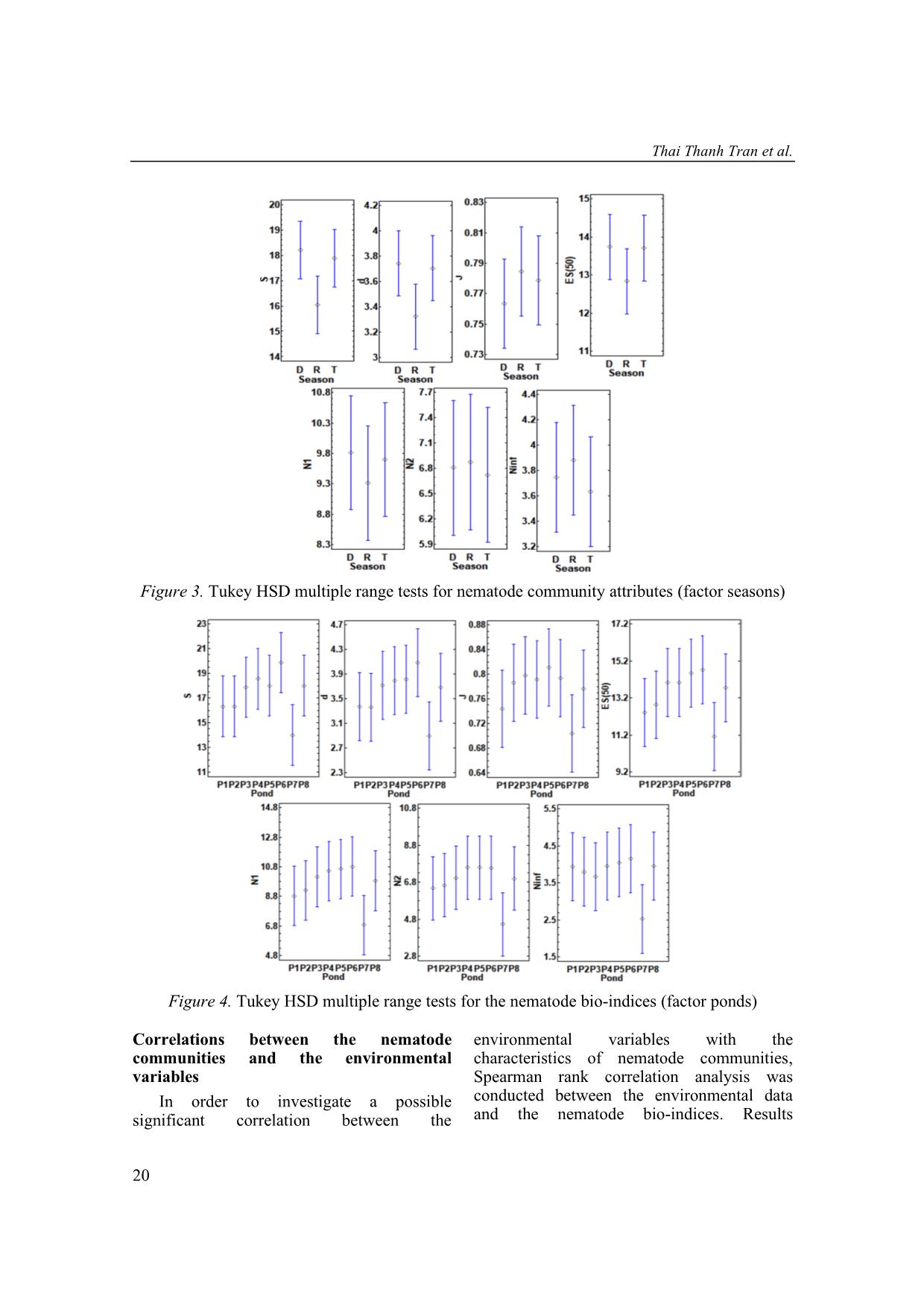
Trang 6
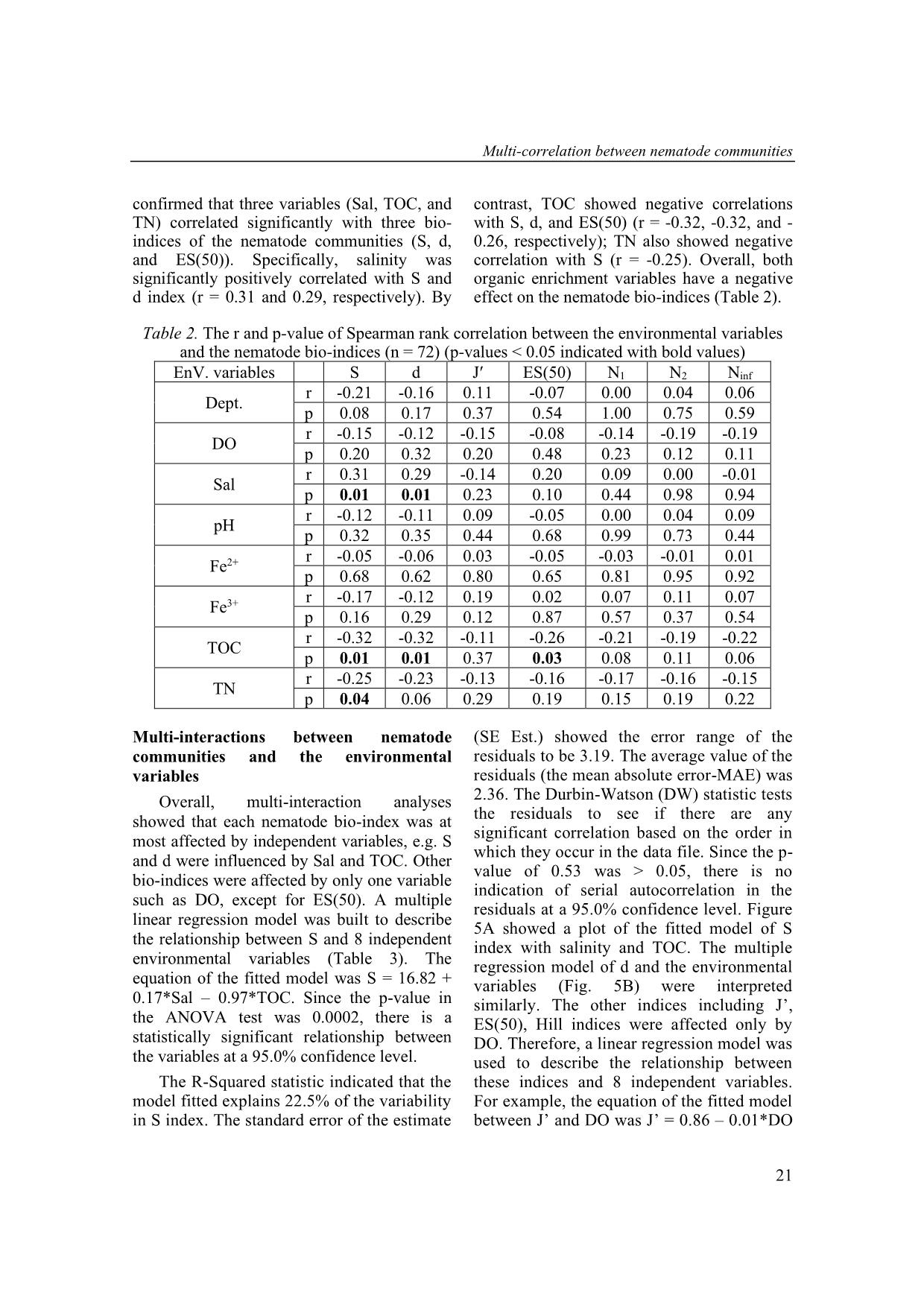
Trang 7
Trang 8
Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Multi-correlation between nematode communities and environmental variables in mangrove-shrimp ponds, Ca Mau province, Southern Vietnam

sms in general. Figure 6. Single and multi-correlation between the nematode bio-indices and the environmental variables Multi-correlation between nematode communities 25 What variables need to be considered to raise biodiversity of nematode communities (shrimp’s food source)? Using the multi-correlation results from this study in combination with other studies (Table 5), high salinity could help promoting nematode diversities, whereas a high value of depth, DO, pH, and organic concentration (TOC, TN) could decrease the diversity. Although nematode diversities can be affected by a number of abiotic variables such as salinity, temperature, hydrodynamics, sediment grain size, oxygenation level and food availability (Ingels et al., 2011; Cai et al., 2012; Ngo et al., 2013a; Zeppilli et al., 2013; Górska et al., 2014), salinity is the most important variable. Several studies showed that salinity is one of the most common ancillary measures used in coastal and marine ecological studies to monitor drivers of benthic assemblages (Alber, 2002; Ysebaert & Herman, 2002; Kimmel & Roman, 2004). Moreover, salinity gradients could be more important in explaining diversity across multiple estuarine systems (Van Diggele, 2016). Therefore, salinity concentration should be considered and regularly monitored in CMMSFP. The optimal salinity for shrimp culture is about 15−25 ppt (Boyd, 1995) which is vital for pond dynamics, although shrimps can be grown in salinities varying from 4 ppt to 26 ppt. Likewise, in an earlier study, P. monodon favored salinity ranging from of 6.5 ppt to 25.5 ppt favored the growth (Das et al., 2001). Table 5. Single-correlation between the nematode bio-indices and the environmental variables form others studies Dep Sal DO pH TN S -[1] +[2, 7] -[3] -[4] -[7] d -[5] +[7] N.A -[4] -[7] H′ -[5, 6] +[2] -[3] -[4] -[3] ES(50) N.A N.A -[3] N.A -[7] N1 -[5] +[2] -[3] -[3] -[3] N2 -[5] N.A -[3] -[3] -[3] Ninf -[5] N.A N.A -[3] -[3] Notes: “+”: Positive correlations; “-”: Negative correlations; N.A: Not available; [1]: Gambi et al. (2003); [2]: Tran et al. (2018b); [3]: Ngo et al. (2016); [4]: Ngo et al. (2013b); [5]: Tran et al. (2017a); [6]: Liu et al. (2015); [7]: This contribution. CONCLUSION This study found significant multi- interaction between nematode communities’ bioindices with environmental variables in the CMMSFPs. The biodiversity of nematode communities have been considered to be high which provided more natural food for shrimps. Furthermore, the multi-correlation between the nematode bio-indices and the environmental variables produced completely different results from those of single- correlation analyses. Although the present study has been able to show the advantage of the multi-correlation, there are still some points we would like to address in future work, especially the complex interactions between the environmental variables and nematode communities. REFERENCES Alber M., 2002. A conceptual model of estuarine freshwater inflow management. Estuaries, 25(6): 1246–1261. Alongi D. M., 1987. Inter-estuary variation and intertidal zonation of free-living nematode communities in tropical mangrove systems. Marine Ecology Progress Series, 40(1): 103–114. Thai Thanh Tran et al. 26 Bezerra T. N., Decraemer W., Eisendle- Flöckner U., Hodda M., Holovachov O., Leduc D., Miljutin D., Mokievsky V., Peña Santiago R., Sharma J., Smol N., Tchesunov A., Venekey V., Zeng Z., Vanreusel, A., 2018. Nemys: World Database of Nematodes. Bosma R. H., Nguyen T. H., Siahainenia A. J., Tran H. T., Tran H. N., 2016. Shrimp‐ based livelihoods in mangrove silvo‐ aquaculture farming systems. Reviews in Aquaculture, 8(1): 43–60. Boyd C. E., 1995. Soil and water quality management in aquaculture ponds. INFOFISH International, 5: 29–36. Ca Mau Province Portal, 2013. Retrieved December 1, 2013, from Cai L., Fu S., Yang J., Zhou X., 2012. Distribution of meiofaunal abundance in relation to environmental variables in Beibu Gulf, South China Sea. Acta Oceanologica Sinica, 31(6): 92–103. Chong V. C. & Sasekumar A., 1981. Food and feeding habits of the white prawn Penaeus merguiensis. Marine ecology progress series, 5(20): 185–191. Das S. K., Saksena D. N., 2001. Farm management and water quality in relation to growth of Penaeus monodon in modified extensive shrimp culture system. Journal of Inland Fisheries Society of India, 33(2): 55–61. De Grisse A. T., 1969. Redescription ou modification de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Meded. Rijksfakulteit Landbouwwetenschappen, Gent 34: 351–369. El Hag E. A., 1984. Food and food selection of the Penaeid prawn Penaeus monodon (Fabricius). In Limnology and Marine Biology in the Sudan (pp. 213–217). Springer, Dordrecht. Fitzgerald W. J., 2000. Integrated mangrove forest and aquaculture systems in Indonesia. In Mangrove-friendly aquaculture: Proceedings of the workshop on mangrove-friendly aquaculture, eds. J.H. Primavera, L.M.B. Garcia, M.T. Castaños, and M.B. Surtida, 21–34. Iloilo City, Philippines: Organized by the Aquaculture Department, SEAFDEC. Gambi C., Vanreusel A., Danovaro, R., 2003. Biodiversity of nematode assemblages from deep-sea sediments of the Atacama Slope and Trench (South Pacific Ocean). Deep Sea Research Part I: Oceanographic Research Papers, 50(1): 103–117. Gee J. M., Somerfield P. J., 1997. Do mangrove diversity and leaf litter decay promote meiofaunal diversity?. Journal of experimental marine Biology and Ecology, 218(1): 13–33. Górska B., Grzelak K., Kotwicki L., Hasemann C., Schewe I., Soltwedel T., Włodarska-Kowalczuk M., 2014. Bathymetric variations in vertical distribution patterns of meiofauna in the surface sediments of the deep Arctic ocean (HAUSGARTEN, Fram strait). Deep Sea Research Part I: Oceanographic Research Papers, 91: 36–49. Gwyther J., 2003. Nematode assemblages from Avicenniamarina leaf litter in a temperate mangrove forest in south- eastern Australia. Marine Biology, 142(2): 289–297. Ha T. T. T., van Dijk H., Bush, S. R., 2012. Mangrove conservation or shrimp farmer's livelihood? The devolution of forest management and benefit sharing in the Mekong Delta, Vietnam. Ocean & Coastal Management, 69: 185–193. Hai T. N., 2005. Effects of mangrove leaf litters on the integrated mangrove–shrimp farming systems in Ca Mau Province, Vietnam. School of Environment, Resources and Development. Asian Institute of Technology, Thailand. Hamilton S., 2013. Assessing the role of commercial aquaculture in displacing Multi-correlation between nematode communities 27 mangrove forest. Bulletin of Marine Science, 89(2): 585–601. Hodda M., Nicholas W. L., 1985. Meiofauna associated with mangroves in the Hunter River estuary and Fullerton Cove, south- eastern Australia. Marine and Freshwater Research, 36(1): 41–50. Ingels J., Tchesunov A. V., Vanreusel A., 2011. Meiofauna in the Gollum Channels and the Whittard Canyon, Celtic Margin- how local environmental conditions shape nematode structure and function. PLoS One, 6(5): e20094. Kimmel D. G., Roman M. R., 2004. Long- term trends in mesozooplankton abundance in Chesapeake Bay, USA: influence of freshwater input. Marine Ecology Progress Series, 267: 71–83. Liu X. S., Xu M., Zhang J. H., Mu G., Liu D., Li X., 2014. Abundance and biomass of deep-sea meiofauna in the northern South China Sea. Journal of Tropical Oceanography, 33(2): 52–59. Liu X., Xu M., Zhang J., Liu D., Li X., 2015. Community structure and biodiversity of free-living marine nematodes in the northern South China Sea. Acta Oceanologica Sinica, 34(6): 77–85. Netto S. A., & Gallucci F., 2003. Meiofauna and macrofauna communities in a mangrove from the Island of Santa Catarina, South Brazil. Hydrobiologia, 505(1−3): 159–170. Ngo X. Q., Chau N. N., & Tu N. D., 2013a. Correlation between nematode communities with some environmental parameters in the Dai estuary, Ben Tre. Journal of Biology, 35(3se): 1–7. Ngo X. Q., Chau, N. N., Nguyen D. T., Pham V. L, Vanreusel A., 2013c. Distribution pattern of free living nematode communities in the eight Mekong estuaries by seasonal factor. Journal Vietnamese Environment, 4(1): 28–33. Ngo X. Q., Chau, N. N., Smol N., Prozorova L., & Vanreusel A., 2016. Intertidal nematode communities in the Mekong estuaries of Vietnam and their potential for biomonitoring. Environmental monitoring and assessment, 188(2): 91–106. Ngo X. Q., Smol N., Vanreusel A., 2013b. The meiofauna distribution in correlation with environmental characteristics in 5 Mekong estuaries, Vietnam. Cahiers de Biologie Marine 54: 71–83. Ngo X. Q., Vanreusel A., Thanh N. V., Smol N., 2007. Biodiversity of meiofauna in the intertidal Khe Nhan mudflat, Can Gio mangrove forest, Vietnam with special emphasis on free living nematodes. Ocean Science Journal, 42(3): 135–152. Nguyen V. T., 2007. Fauna of Vietnam. Free- living nematodes orders Monhysterida, Araeolaimida, Chromadorida, Rhabditida, Enoplida, Mononchida and Dorylaimida. No.22, Science and Technics Publishing House, 455 pp. Pham T. L., Tran T. T., Nguyen T. M. Y., Ngo X. Q., 2017. Phytoplankton community in integrated shrimp-mangrove farming ponds in Ca Mau Province. Proceedings of the 7th National Scientific Conference on Ecology and Biological Resources Hanoi,793–800. (in Vietnamese). Phan N. H., San H. T., 1993. Mangroves of Vietnam, IUCN Wetlands Programme, Bangkok, Thailand. Platt H. M., Warwick R. M., 1983. Free-living Marine Nematodes (Part I. British Enoplids). Synopses of the British Fauna No. 28, Linnean Society of London/Estuarine & Brackish Water Society. Platt H. M., Warwick R. M., 1988. Free-living Marine Nematodes (Part II. British Chromadorids). Linnean Society of London and the Estuarine and Brackish- Water Sciences Association. Primavera J. H., 2000. Development and conservation of Philippine mangroves: institutional issues. Ecological Economics, 35(1): 91–106. Thai Thanh Tran et al. 28 Richards D. R., Friess, D. A., 2016. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proceedings of the National Academy of Sciences, 113(2): 344–349. Rzeznik-Orignac J., Fichet D., Boucher G., 2003. Spatio-temporal structure of the nematode assemblages of the Brouage mudflat (Marennes Oléron, France). Estuarine, Coastal and Shelf Science, 58(1): 77–88. Semprucci F., Balsamo M., 2012. Free-living marine nematodes as bioindicators: past, present and future perspectives. Environmental Research Journal, 6(1): 17–35. Semprucci F., Moreno M., Sbrocca S., Rocchi M., Albertelli G., Balsamo M., 2013. The nematode assemblage as a tool for the assessment of marine ecological quality status: a case-study in the Central Adriatic Sea. Mediterranean Marine Science, 14(1): 48–57. Takashima F., 2000. Silvofishery: An aquaculture system harmonized with the environment. In Mangrove-friendly aquaculture: Proceedings of the workshop on mangrove-friendly aquaculture, eds. J. H. Primavera, L. M. B. Garcia, M. T. Castaños, and M. B. Surtida, 13–19. Iloilo City, Philippines: SEAFDEC, Organized by the Aquaculture Department. Tho N., Ut V. N., Merckx R., 2011. Physico‐ chemical characteristics of the improved extensive shrimp farming system in the Mekong Delta of Vietnam. Aquaculture Research, 42(11): 1600–1614. Tran T. T., Lam N. L. Q., Quang N. X., Hieu H. H., 2018a. Seasonal and spatial variations of meiofauna communities in correlation to environmental characteristics in the organic shrimp farms of Tam Giang commune, Nam Can district, Ca Mau Province. VNU Journal of Science: Natural Sciences and Technology, 34(1): 55–64. Tran T. T., Le Q. L. N., Le H. D., Nguyen T. M. Y., Ngo X. Q., 2018b. Intertidal meiofaunal communities in relation to salinity gradients in the Ba Lai river, Vietnam. Journal of Vietnamese Environment, 10(2): 138–150. Tran T. T., Nguyen T. M. Y., Ngo X. Q., Pham T. L., 2017a. Effect of different water column depths on nematode communities in the mangrove-shrimp farming system, Ca Mau Province, Vietnam Journal of Marine Science and Technology, 17(4A): 269–278. Tran T. T., Nguyen T. M. Y., Ngo X. Q., Truong T. N., Nguyen N. S., 2017b. Diversity assessment of benthic macroinvertebrate in shrimp-mangro farming ponds, Ca Mau Province Proceedings of the 7th national scientific conference on ecology and biological resources. Science and Technics Publishing House. (in Vietnamese). Tran T. T., Nguyen T. M. Y., Nguyen T., Ngo X. Q., 2017c. Meiofauna in the mangrove- shrimp farms ponds, Ca Mau Province. Journal of Science and Technology, 55(3): 271–284. Tran T. T., Pham T. L., Nguyen T., Ngo X. Q., 2018c. Relationship of free-living nematode communities to some environmental variables in an organic shrimp farms, Ca Mau Province. Journal of Science and Technology, 56(5): 526–648. Truong T. D., & Do L. H., 2018. Mangrove forests and aquaculture in the Mekong river delta. Land use policy, 73: 20–28. Van Diggele, A. D. & Montagna P. A., 2016. Is salinity variability a benthic disturbance in estuaries? Estuaries and Coasts, 39(4): 967–980. Vincx M., 1996. Meiofauna in marine and freshwater sediments. In G. S. Hall (Ed.), Methods for the examination of organismal diversity in soils and sediments (pp. 187–195). Wallinfort, UK: CAB International. Multi-correlation between nematode communities 29 Vu T. P., 2004. National Report on Mangrove in South China Sea. Vietnam Research Centre for Forest Ecology and Environment, Vietnam. Warwick R. M., Platt H. M., Somerfield P. J., 1988. Free living marine nematodes (Part III. Monhysterids). The Linnean Society of London and the Estuarine and Coastal Sciences Association, London. Ysebaert T., Herman, P. M. J., 2002. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Marine Ecology Progress Series, 244: 105–124. Zeppilli D., Bongiorni L., Cattaneo A., Danovaro R., Santos R. S., 2013. Meiofauna assemblages of the Condor Seamount (North-East Atlantic Ocean) and adjacent deep-sea sediments. Deep Sea Research Part II: Topical Studies in Oceanography, 98: 87–100. Zullini A., 2005. The Identification manual for freshwater nematode genera, Lecture book, MSc Nematology Ghent University.
File đính kèm:
 multi_correlation_between_nematode_communities_and_environme.pdf
multi_correlation_between_nematode_communities_and_environme.pdf

