Assessing changes in ecological quality status of sediment in Tri An reservoir (Southeast Vietnam) by using indicator of nematode communities
Abstract: Nematode communities in Tri An Reservoir (Dong Nai Province, Southeast Vietnam)
were explored in the dry season (March) and pre-rainy season (July) of 2019 and analyzed to
evaluate their usage as bioindicators for ecological quality status of sediment. Nematode
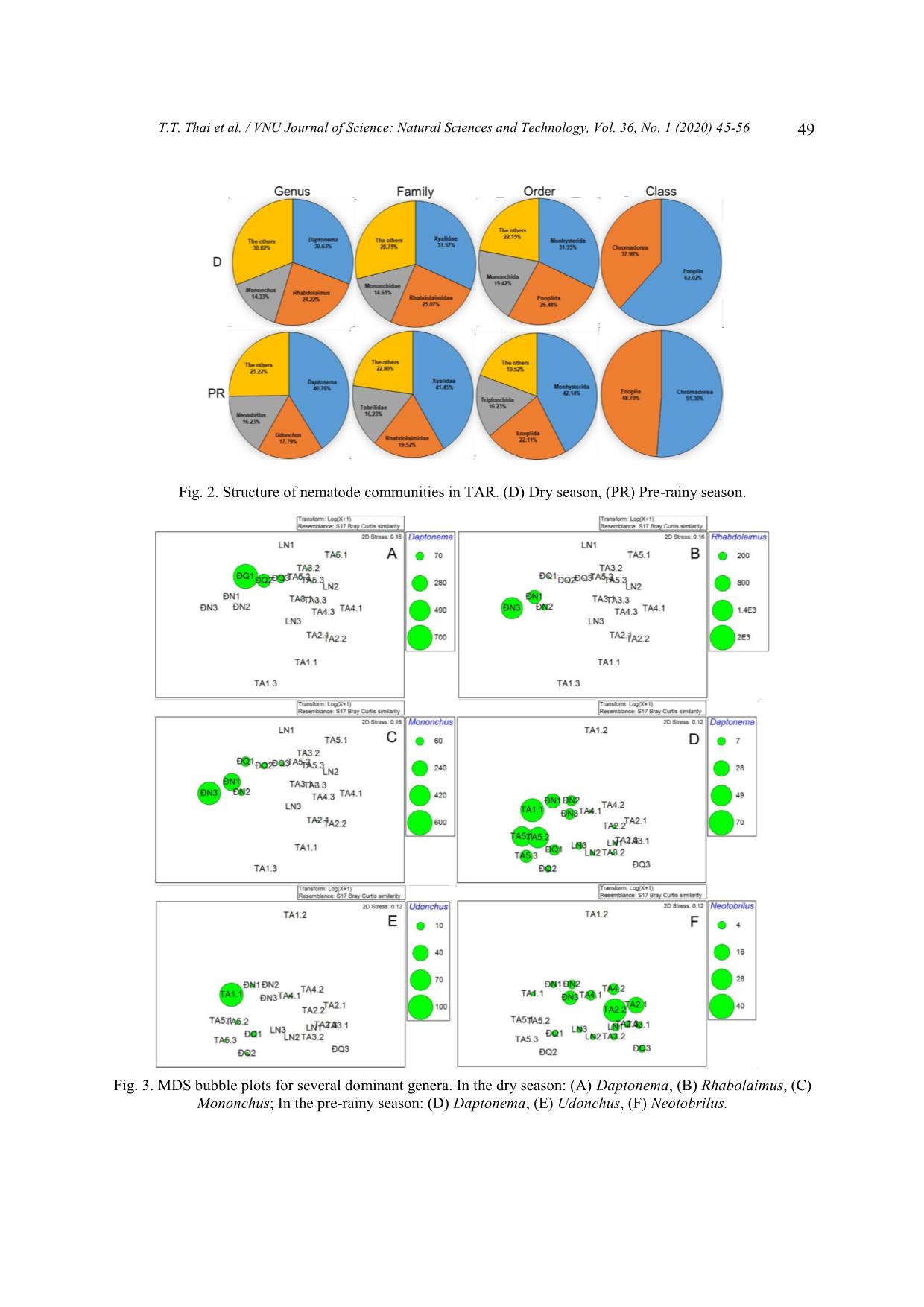
communities consisted of 23 genera belonging to 19 families, 8 orders for the dry and 24 genera, 17
families, 8 orders for the pre-rainy season. Several genera dominated in Tri An Reservoir such as
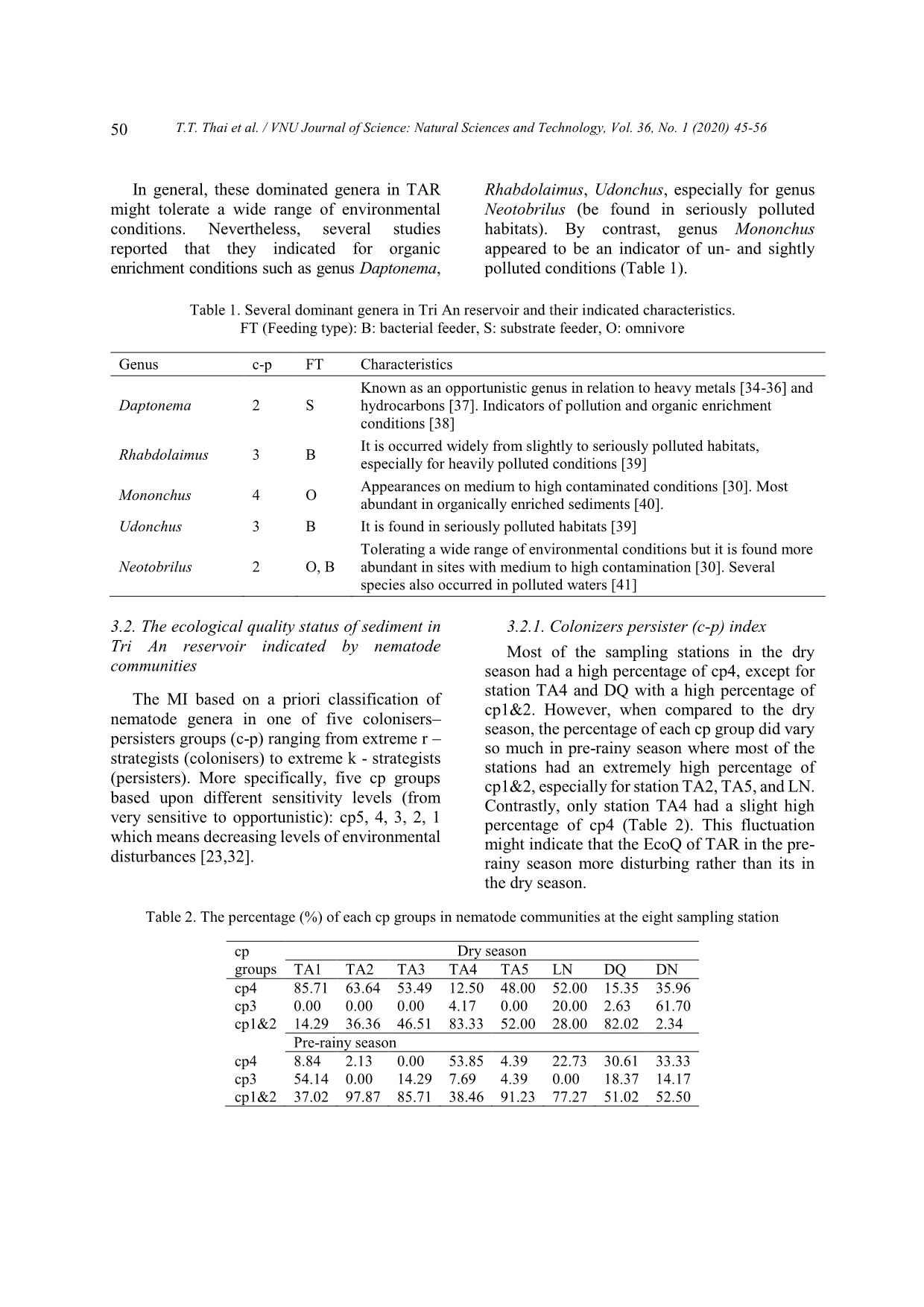
Daptonema, Rhabdolaimus, Udonchus, and Neotobrilus indicated for organic enrichment
conditions. The percentage of cp3&4 and MI (Maturity Index) value in the dry season was higher
than that in the pre-rainy season expressed the ecological quality status of sediment in the dry season
were better than those in the pre-rainy season. Furthermore, the result revealed that MI and c-p%
composition can be used to evaluate the ecological quality status of sediment efficiently.

Trang 1

Trang 2

Trang 3

Trang 4
Trang 5
Trang 6
Trang 7
Trang 8

Trang 9

Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Assessing changes in ecological quality status of sediment in Tri An reservoir (Southeast Vietnam) by using indicator of nematode communities

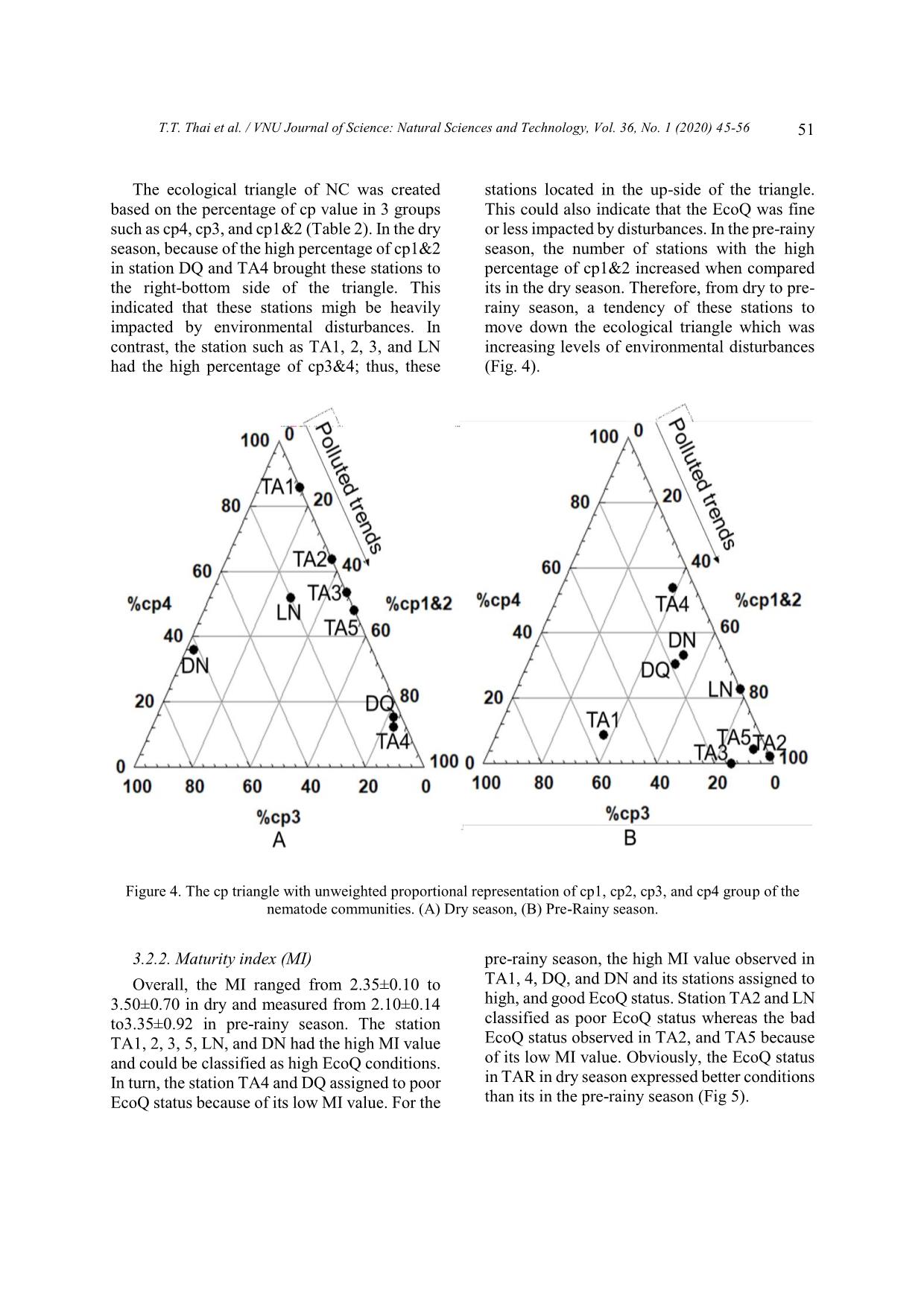
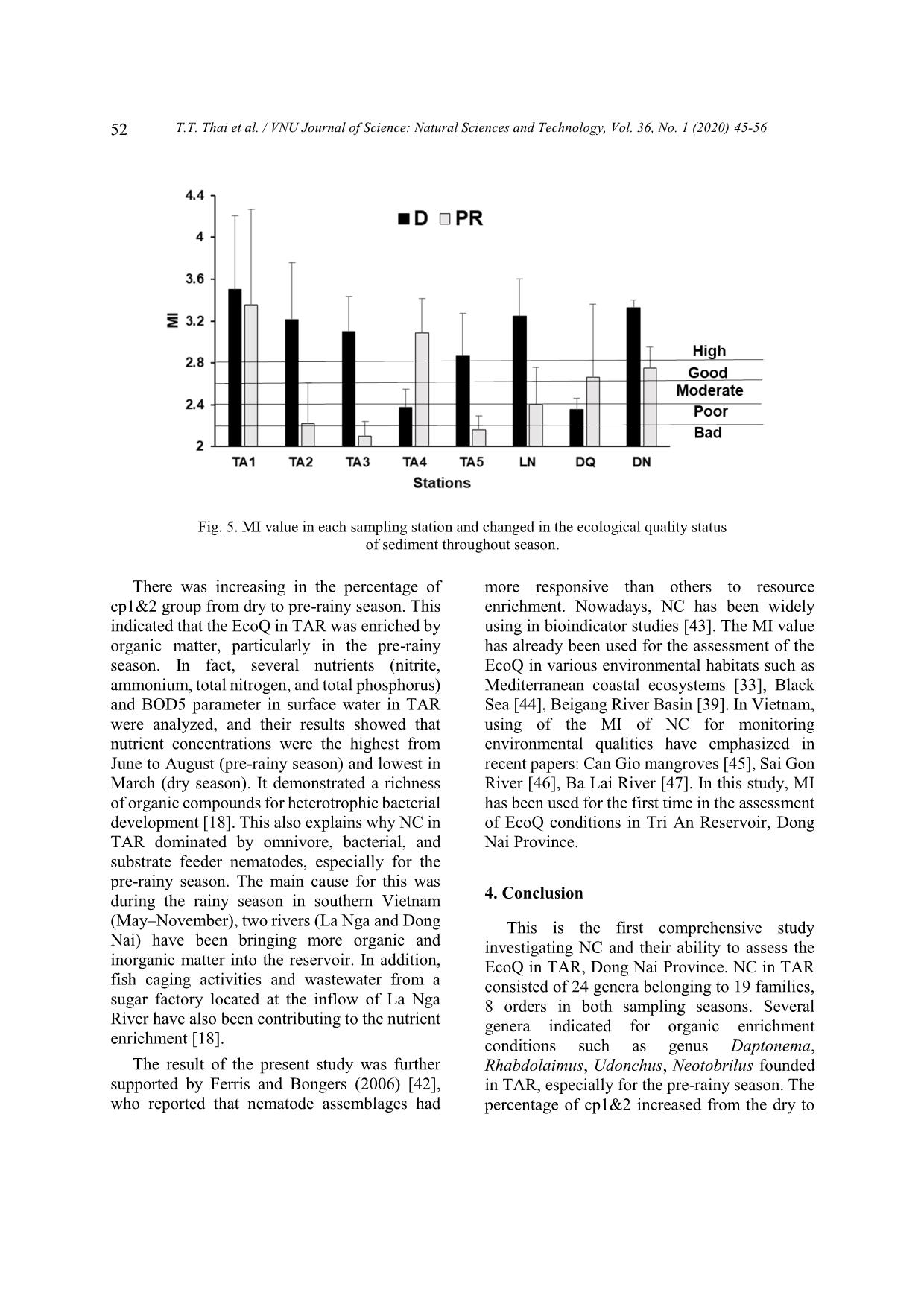
[33], Black Sea [44], Beigang River Basin [39]. In Vietnam, using of the MI of NC for monitoring environmental qualities have emphasized in recent papers: Can Gio mangroves [45], Sai Gon River [46], Ba Lai River [47]. In this study, MI has been used for the first time in the assessment of EcoQ conditions in Tri An Reservoir, Dong Nai Province. 4. Conclusion This is the first comprehensive study investigating NC and their ability to assess the EcoQ in TAR, Dong Nai Province. NC in TAR consisted of 24 genera belonging to 19 families, 8 orders in both sampling seasons. Several genera indicated for organic enrichment conditions such as genus Daptonema, Rhabdolaimus, Udonchus, Neotobrilus founded in TAR, especially for the pre-rainy season. The percentage of cp1&2 increased from the dry to T.T. Thai et al. / VNU Journal of Science: Natural Sciences and Technology, Vol. 36, No. 1 (2020) 45-56 53 the pre-rainy season. Moreover, the MI value in the dry was higher rather than that in the pre- rainy season. This indicated that the EcoQ conditions in the dry were better than those in the pre-rainy season. Moreover, the EcoQ in TAR was enriched by organic matter, particularly in the pre-rainy season. The result also suggested that nematodes might be useful indicators for biomonitoring studies in freshwater habitats. However, the future study should pay more experience from field studies and laboratory experiments in order to better interpret changes in the composition of NC, and thus to be able to use sophisticated indices, such as the MI, for ecological quality status assessment of sediment. Acknowledgment This research was funded by the Vietnam Academy of Science and Technology (VAST) under grant number KHCBSS.02/19-21. The author thanks the staff of the Department of Environmental Management and the Department of Biological Resources (ITB-VAST) for precious help with laboratory analyses. References [1] N.M. Hayes, B.R. Deemer, J.R. Corman, N.R. Razavi, K.E. Strock, Key differences between lakes and reservoirs modify climate signals: A case for a new conceptual model, Limnology and Oceanography Letters 2(2) (2017) 47-62. https:// doi.org/10.1002/lol2.10036. [2] R.J. Hogeboom, L. Knook, A. Y. Hoekstra, The blue water footprint of the world's artificial reservoirs for hydroelectricity, irrigation, residential and industrial water supply, flood protection, fishing and recreation, Advances in water resources 113 (2018) 285-294. https://doi. org/10.1016/j.advwatres.2018.01.028. [3] R.G. Wetzel, Limnology: Lake and river ecosystems (3rd edition), Elsevier Science, San Diego, 2001. [4] D.E. Gernaat, P.W. Bogaart, D.P. van Vuuren, H. Biemans, R. Niessink, High-resolution assessment of global technical and economic hydropower potential, Nature Energy 2(10) (2017) 821. https:// doi.org/10.1038/s41560-017-0006-y. [5] K. Timpe, D. Kaplan, The changing hydrology of a dammed Amazon, Science Advances 3(11) (2017) e1700611. https://doi.org/10.1126/sciadv. 1700611. [6] L. Zeng, L. Zhou, D.L. Guo, D.H. Fu, P. Xu, S. Zeng, Q.D. Tang, A.L. Chen, F.Q. Chen, Y. Luo, G.F. Li, Ecological effects of dams, alien fish, and physiochemical environmental factors on homogeneity/heterogeneity of fish community in four tributaries of the Pearl River in China, Ecology and Evolution 7(11) (2017) 3904-3915. https:// doi.org/10.1002/ece3.2920. [7] R. Freeman, W. Bowerman, T. Grubb, A. Bath, G. Dawson, K. Ennis, J. Giesy, Opening rivers to Trojan fish: The ecological dilemma of dam removal in the Great Lakes, Conservation in Practice 3 (2002) 35-40. https://doi.org/10.1111/j. 1526-4629.2002.tb00045.x. [8] W.W. Carmichael, Cyanobacteria secondary metabolites-the cyanotoxins, Journal of Applied Bacteriology 72(6) (1992) 445-459. https://doi. org/10.1111/j.1365-2672.1992.tb01858.x. [9] Carew-Reid, Jeremy, K. Josh, C. Alison, Biodiversity and Development of the Hydropower Sector: Lessons from the Vietnamese Experience -Volume I, in: Review of the Effects of Hydropower Development on Biodiversity in Viet Nam, ICEM-International Centre for Environmental Management, Prepared for the Critical Ecosystem Partnership Fund Hanoi Viet Nam (2010). [10] G. Berkamp, M. McCartney, P. Dugan, J. McNeely, M. Acreman, Dams, Ecosystem Functions and Environmental Restoration Thematic Review II.1 prepared as an input to the World Commission on Dams, Cape Town, 2000. [11] D.T. Ngo, C. Kieu, M. Thatcher, D. Nguyen-Le, T. Phan-Van, Climate projections for Vietnam based on regional climate models, Climate Research 60(3) (2014) 199-213. https://doi.org/ 10.3354/cr01234. [12] J. Soussan, Strategic Environmental Assessment of Hydropower in the context of the Power Development Plan VI in Vietnam: Final report. SEI/ADB GMS Joint Publication, 2008. [13] Vietnam Ministry Of Science, Technology and Environment, The valuable biodiversity and environment wetlands in Vietnam, Agricultural Publishing House Hanoi, 2001. [14] T.S. Dao, G. Cronberg, J. Nimptsch, L.C. Do- Hong, C. Wiegand, Toxic cyanobacteria from Tri An Reservoir, Vietnam, Nova Hedwigia 90(3-4) (2010) 433-448. https://doi.org/10.1127/0029- 5035/2010/0090-0433. T.T. Thai et al. / VNU Journal of Science: Natural Sciences and Technology, Vol. 36, No. 1 (2020) 45-56 54 [15] N.V. Hoang, D.V. Thuan, N.D. Roi, Study on the impact of the Tri An reservoir on its downstream groundwater level regime, Vietnam Journal of Earth Sciences 34(4) (2012) 465-476. https://doi. org/10.15625/0866-7187/34/4/2806 (in Vietnamese). [16] M.T. Tan, D.V. Thuan, V.V. Ha, N.T. Tan, T.T.T. Ha, N.V. Tao, N.C. Quan, Assessment of sedimentation in Tri An reservoir by nuclear technique, geological analyses and GIS, Vietnam Journal of Earth Sciences 36(1) (2014) 51-60. https://doi.org/10.15625/0866-7187/36/1/4141. [17] MoF, Fisheries resources of Vietnam Agricultural Publishing House Hanoi, 1996 (in Vietnamese), [18] T.S. Dao, J. Nimptsch, C. Wiegand, Dynamics of cyanobacteria and cyanobacterial toxins and their correlation with environmental parameters in Tri An Reservoir, Vietnam, Journal of Water and Health 14(4) (2016) 699-712. https://doi.org/10. 2166/wh.2016.257. [19] Z.Q. Zhang, Animal biodiversity: an update of classification and diversity in 2013, Zootaxa 3703(1) (2013) 5-11. https://doi.org/10.11646/zootaxa.37 03.1.3. [20] P.A. Montagna, J.E. Bauer, D. Hardin, R.B. Spies, Meiofaunal and microbial trophic interactions in a natural submarine hydrocarbon seep, Oceanographic Literature Review 10(42) (1995) 882. [21] F. Semprucci, M. Moreno, S. Sbrocca, M. Rocchi, G. Albertelli, M. Balsamo, The nematode assemblage as a tool for the assessment of marine ecological quality status: a case-study in the Central Adriatic Sea, Mediterranean Marine Science 14(1) (2013) 48-57. https://doi.org/10. 12681/mms.366. [22] M. Majdi, W. Traunspurger, Free-living nematodes in the freshwater food web: a review, Journal of Nematology 47(1) (2015) 28. [23] T. Bongers, The maturity index: an ecological measure of environmental disturbance based on nematode species composition, Oecologia 83 (1990) 14–19. [24] H. Ferris, T. Bongers, R.G.M. de Goede, A framework for soil food web diagnostics: extension of the nematode faunal analysis concept, Applied Soil Ecology 18 (2001) 13-29. https://doi. org/10.1016/S0929-1393(01)00152-4. [25] DNFC, Assessment of potentials, current situation and planning for sustainable fisheries development in Tri An reservoir, Department of Agriculture and Rural Development Dong Nai, 1997 (in Vietnamese). [26] DNFC, Annual technical reports on fisheries activities of Tri An reservoir from 1987-2005, Department of Agriculture and Rural Development, Dong Nai, 2005 (in Vietnamese). [27] V.C. Luong, Y. Yi, C.K. Lin, Cove culture of marble goby (Oxyeleotris marmorata Bleeker) and carps in Tri An Reservoir of Vietnam, Aquaculture 244(1-4) (2005) 97-107. https://doi.org/10.1016/j.aquaculture.2004.10.027. [28] M. Vincx, Meiofauna in marine and freshwater sediments, in: G. S. Hall (Eds.), Methods for the examination of organismal diversity in soils and sediments, Wallinfort UK, 1996. [29] A.T. De Grisse, Redescription ou modifications de quelques technique utilis [a] es dan l'etude des n [a] ematodes phytoparasitaires, 1969. [30] E. Abebe, I. Andrássy, W. Traunspurger, Freshwater Nematodes: Ecology and Taxonomy, CABI, 2006. [31] A. Zullini, The Identification manual for freshwater nematode genera, Lecture book, MSc Nematology Ghent University, 2005. [32] T. Bongers, R. Alkemade, G.W. Yeates, Interpretation of disturbance induced maturity decrease in marine nematode assemblages by means of the Maturity Index, Marine Ecology Progress Series 76 (1991) 135-142. [33] M. Moreno, F. Semprucci, L. Vezzulli, M. Balsamo, M. Fabiano, G. Albertelli, The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems, Ecological Indicators 11(2) (2011) 328-336. https://doi.org/ 10.1016/j.ecolind.2010.05.011. [34] R.N. Millward, A. Grant, Assessing the impact of copper on nematode communities from a chronically metal metal-enriched estuary using pollutioninduced community tolerance, Marine Pollution Bulletin 30 (1995) 701-706. https://doi. org/10.1016/0025-326X(95)00053-P. [35] A. Hedfi, E. Mahmoudi, F. Boufahja, H. Beyrem, P. Aïssa, Effects of increasing levels of nickel contamination on structure of offshore nematode communities in experimental microcosms, Bulletin of Environmental Contaminant and Toxicology 79 (2007) 345-349. https://doi.org/10. 1007/s00128-007-9261-0. [36] F. Boufahja, A. Hedfi, J. Amorri, P. Aïssa, H. Beyrem, E. Mahmoudi, An assessment of the impact of chromium-amended sediment on a marine nematode assemblage using microcosm bioassays, Biological Trace Elements Research 142 (2011) 242-255. https://doi.org/10.1007/ s12011-010-8762-6. [37] E. Mahmoudi, N. Essid, H. Beyrem, A. Hedfi, F. Boufahja, P. Vitiello, P. Aïssa, Effects of hydrocarbon contamination on a free living marine nematode community: results from microcosm experiments, Marine Pollution Bulletin 50 (2005) 1197-1204. . https://doi.org/10. 1016/j.marpolbul.2005.04.018. T.T. Thai et al. / VNU Journal of Science: Natural Sciences and Technology, Vol. 36, No. 1 (2020) 45-56 55 [38] A.S. Alves, H. Adão, T.J. Ferrero, J.C. Marques, M. J. Costa, J. Patrício, Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: the use of nematodes in ecological quality assessment, Ecological Indicators 24 (2013) 462-475. https://doi.org/10.1016/j.ecolind. 2012.07.013. [39] H.C. Wu, P.C. Chen, T.T. Tsay, Assessment of nematode community structure as a bioindicator in river monitoring, Environmental Pollution 158(5) (2010) 1741-1747. https://doi.org/10.1016/j.envpol. 2009.11.015. [40] R. Niemann, M. Arens, K. Koczwara, D. Sturhan, Untersuchungen über die Eignung von Nematoden zur Gütebewertung von Flieβgewässern, Mitteilungen der biologischen Bundesanstalt für Land-Forstwirtschaft, Berlin-Dahlem 317 (1996) 195–208. [41] A.H. Arthington, G.W. Yeates, D.L. Conrick, Nematodes, including a new record of Tobrilus diversipapillatus in Australia, as potential indicators of sewage effluent pollution, Australian Journal of Marine and Freshwater Research 37 (1986) 159-166.https://doi.org/10.1071/MF9860159. [42] H. Ferris, T. Bongers, Nematode indicators of organic enrichment, Journal of Nematology 38(1) (2006) 3-12. [43] X.Q. Ngo, N.C. Nguyen, M. Nic, P. Larisa, V. Ann, Intertidal nematode communities in the Mekong estuaries of Vietnam and their potential for biomonitoring, Environmental Monitoring and Assessment 188(2) (2016) 1-16. https://doi.org/ 10.1007/s10661-016-5091-z. [44] D. Ürkmez, M. Sezgin, L. Bat, Use of nematode Maturity Index for the determination of ecological quality status: a case study from the Black Sea, Journal of Black Sea/Mediterranean Environment 20(2) (2016) 96-107. [45] T.L. Ngo, X.Q. Ngo, Using the Maturity Index (MI) of nematode communities as bioindicator to assess water quality in Khe Đoi and Wastewater waterways in the Can Gio mangrove forest, Ho Chi Minh City, Journal Science 47(81) (2013) 132 – 141 (in Vietnamese). [46] T.M.Y. Nguyen, X.Q. Ngo, Rapid assessment of sediment environmental quality in the Sai Gon River harbors by applying MI index and cp triangle of free living nematodes, the proceeding of International workshop on environment and climate change – challenge, response and lesson learnt (2015) 95-102. [47] T.T. Tran, Q.L. Nguyen Le, T.M.Y. Nguyen, N.S. Hoang, N.X. Ngo, Nematode communities as a tool for the assessment of ecological quality status of sediment: the case of Ba Lai river, Ben Tre province, Journal of Biotechnology 15(3A) (2017) 295-302. T.T. Thai et al. / VNU Journal of Science: Natural Sciences and Technology, Vol. 36, No. 1 (2020) 45-56 56 Appendix Appendix 1. Structure of nematode communities in Tri An Reservoir Genus Family Order Class Dry P-Rainy Achromadora Achromadoridae Chromadorida Chromadorea + + Aphanonchus Aphanolaimidae Plectida Chromadorea + Aphelenchoides Aphelenchoididae Rhabditida Chromadorea + Cephalobus Cephalobidae Rhabditida Chromadorea + Chromadorita Chromadoridae Chromadorida Chromadorea + + Cobbonchus Mononchidae Mononchida Enoplia + + Cryptonchus Cryptonchidae Mononchida Enoplia + + Daptonema Xyalidae Monhysterida Chromadorea + + Dichromadora Chromadoridae Chromadorida Chromadorea + Goffartia Diplogastridae Rhabditida Chromadorea + Ironus Ironidae Enoplida Enoplia + + Mesodorylaimus Dorylaimidae Dorylaimida Enoplia + + Mononchulus Mononchulidae Mononchida Enoplia + + Mononchus Mononchidae Mononchida Enoplia + + Monshystera Monhysteridae Monhysterida Chromadorea + + Monshystrella Monhysteridae Monhysterida Chromadorea + + Mylonchulus Mylonchulidae Mononchida Enoplia + + Neotobrilus Tobrilidae Triplonchida Enoplia + + Paractinolaimus Actinolaimidae Dorylaimida Enoplia + Paracyatholaimus Cyatholaimidae Chromadorida Chromadorea + Paraplectonema Leptolaimidae Plectida Chromadorea + + Plectus Plectidae Plectida Chromadorea + Ptycholaimelus Chromadoridae Chromadorida Chromadorea + Prismatolaimus Prismatolaimidae Triplonchida Enoplia + Rhabditidoides Diplogastridae Rhabditida Chromadorea + + Rhabdolaimus Rhabdolaimidae Enoplida Enoplia + + Theristus Xyalidae Monhysterida Chromadorea + + Terschellinia Linhomoeidae Monhysterida Chromadorea + Udonchus Rhabdolaimidae Enoplida Enoplia + +
File đính kèm:
 assessing_changes_in_ecological_quality_status_of_sediment_i.pdf
assessing_changes_in_ecological_quality_status_of_sediment_i.pdf

