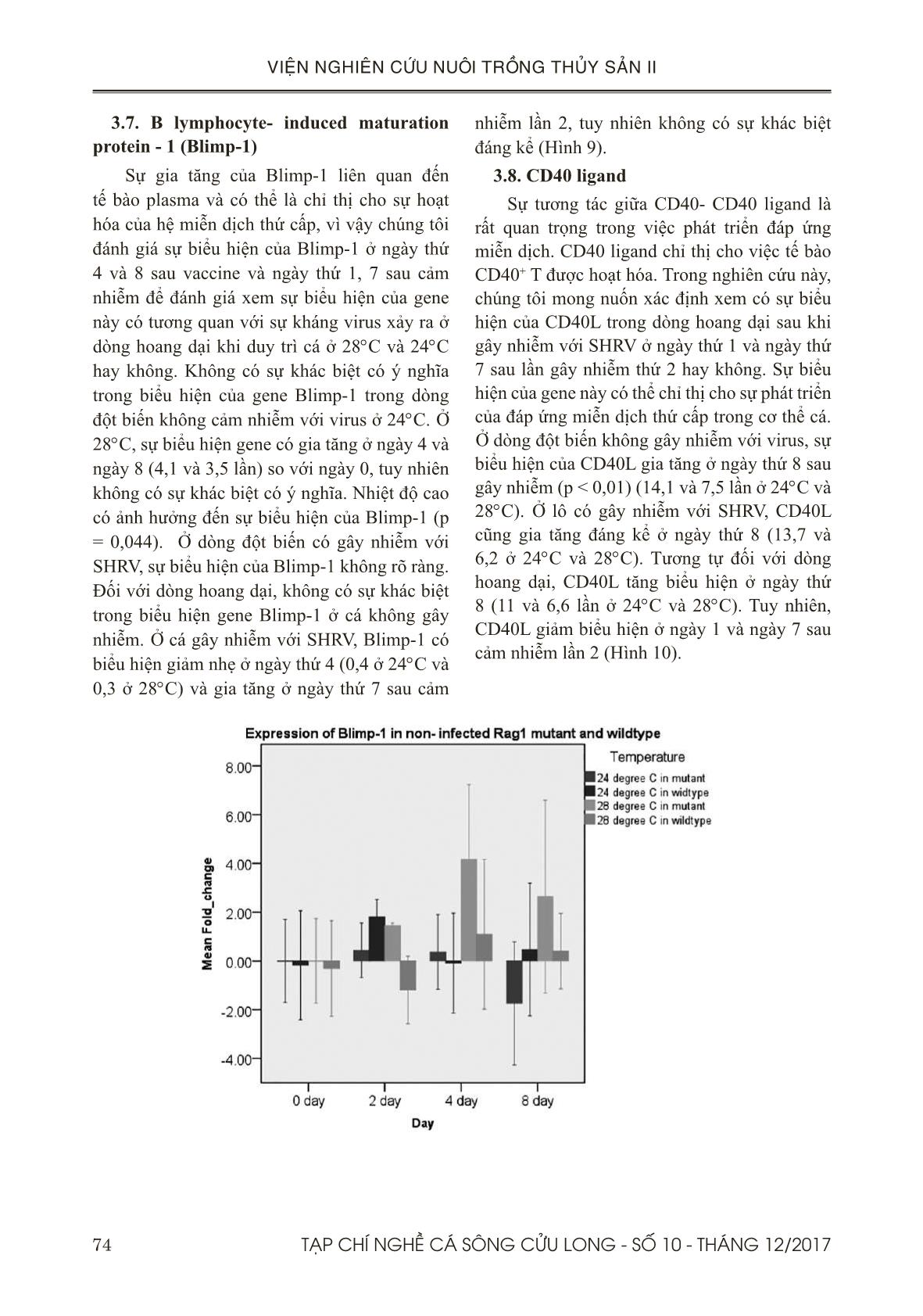
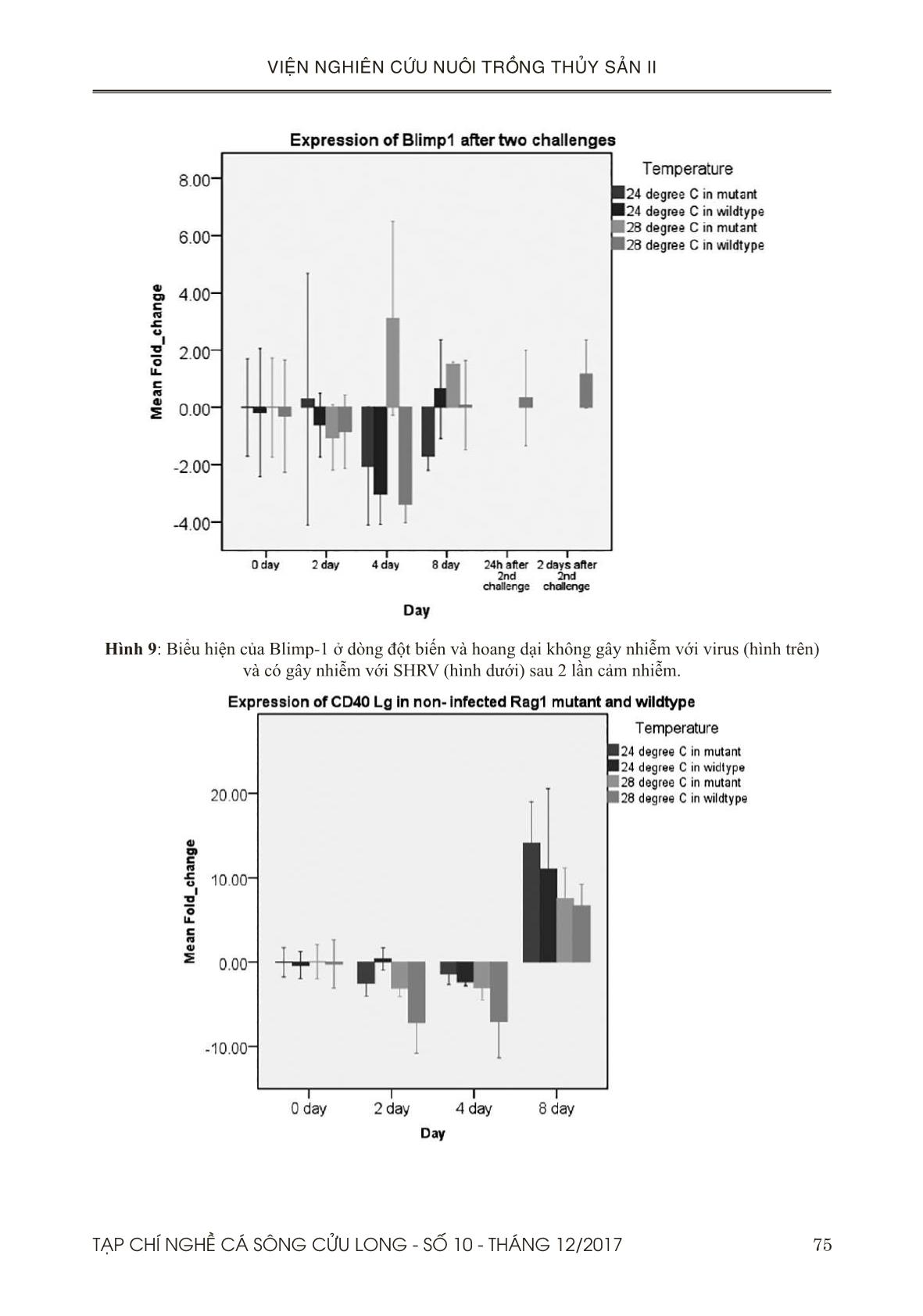
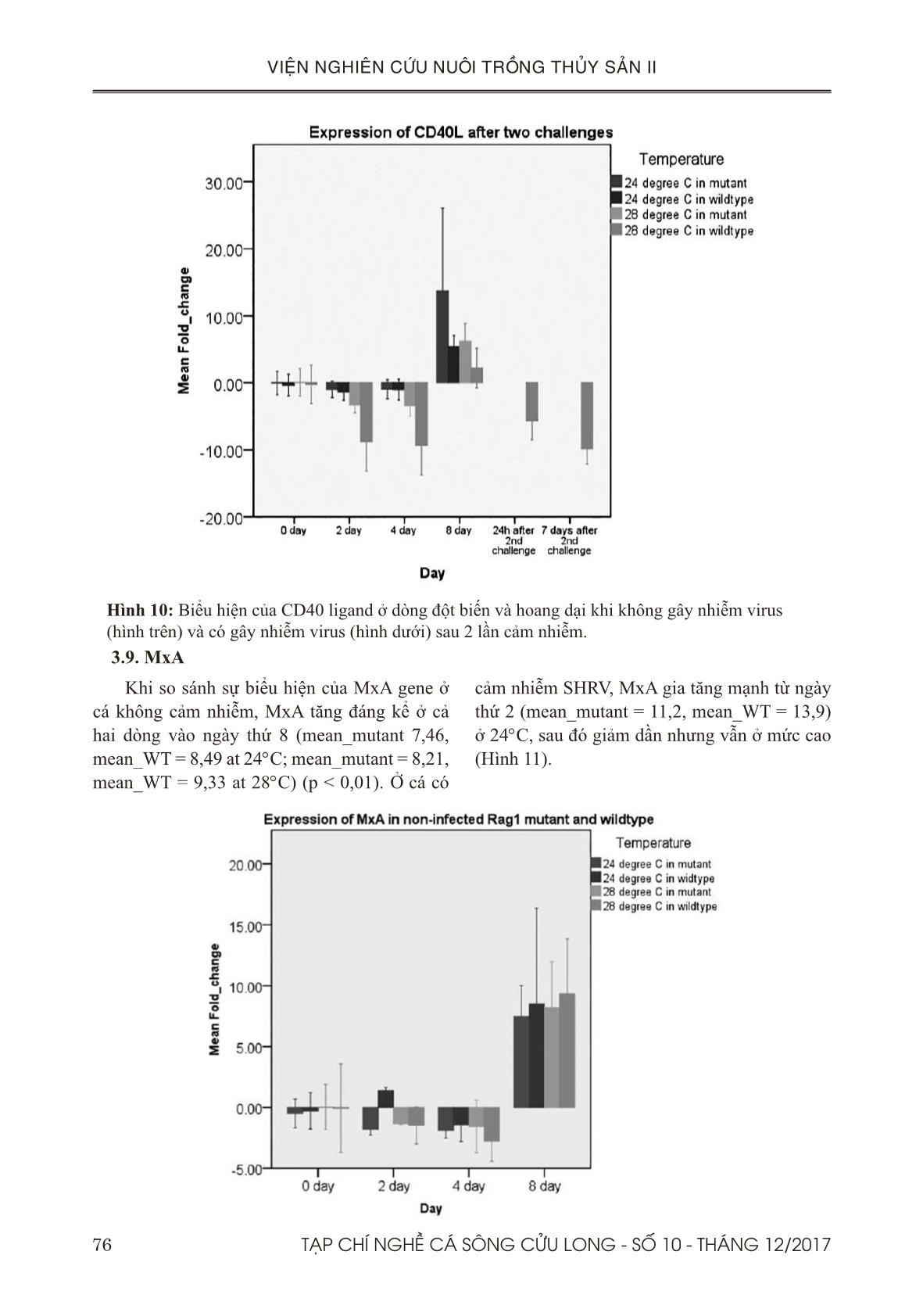
Khác biệt trong sự mẫn cảm với Novirhabdo virus của Zebrafish dòng hoang dại và đột biến và vai trò của lymphocytes
Để nghiên cứu sự đóng góp của miễn dịch sơ cấp và lymphocyte dựa trên miễn dịch kháng lại virus
nhiễm trên cá, hai dòng zebrafish hoang dại (wild-type) và đột biến (Rag1 mutant, không có tế bào
lymphocyte T và B) được cảm nhiễm với Snakehead Rhabdovirus (SHRV), thuộc novirhabdovirus,
bằng cách tiêm xoang bụng (IP) vào cá ở các độ tuổi khác nhau và nhiệt độ khác nhau. Cả hai dòng
đều mẫn cảm cao với SHRV. Cá bệnh có biểu hiện lồi mắt, xuất huyết gốc vây và thân, vảy dựng.
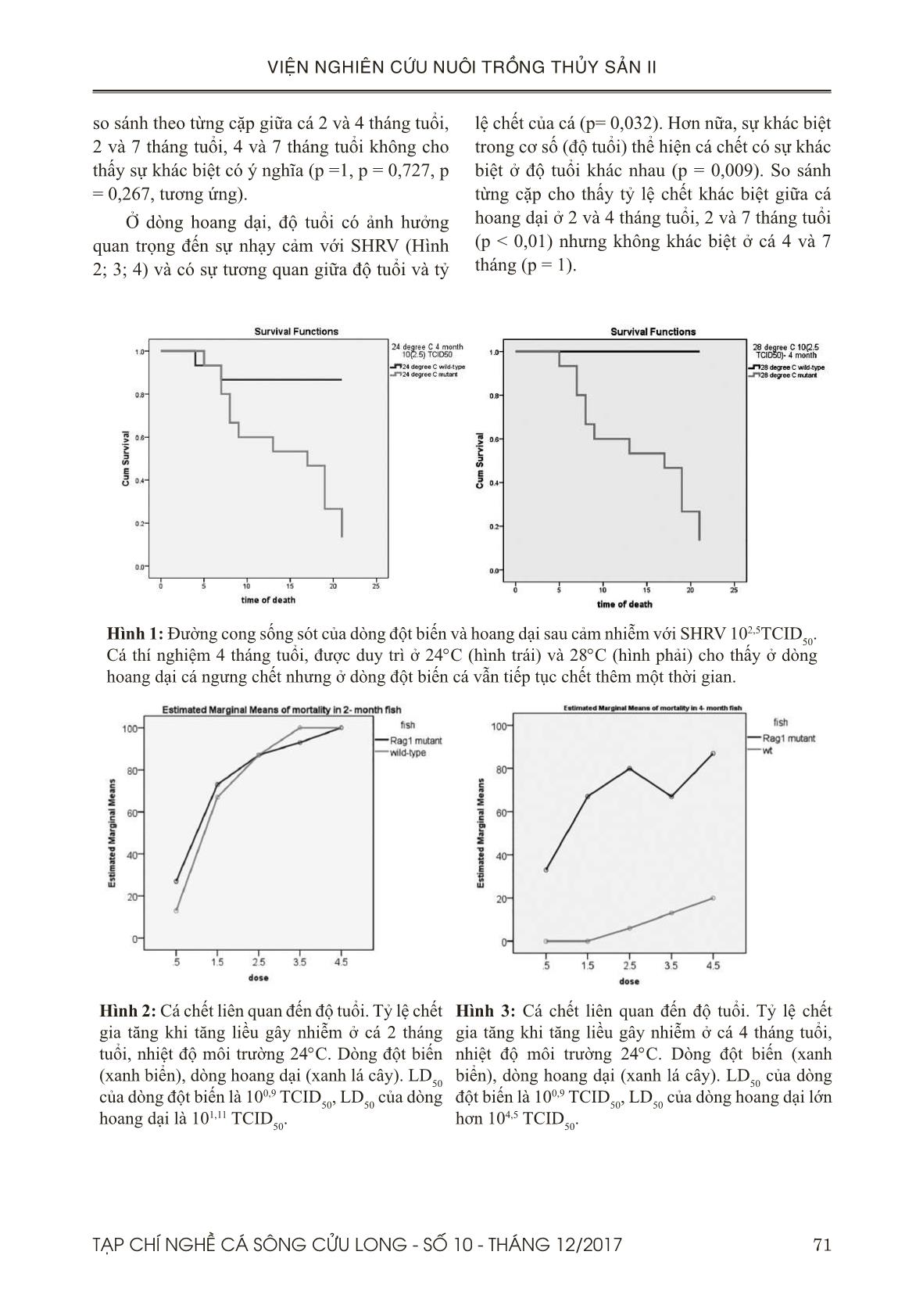
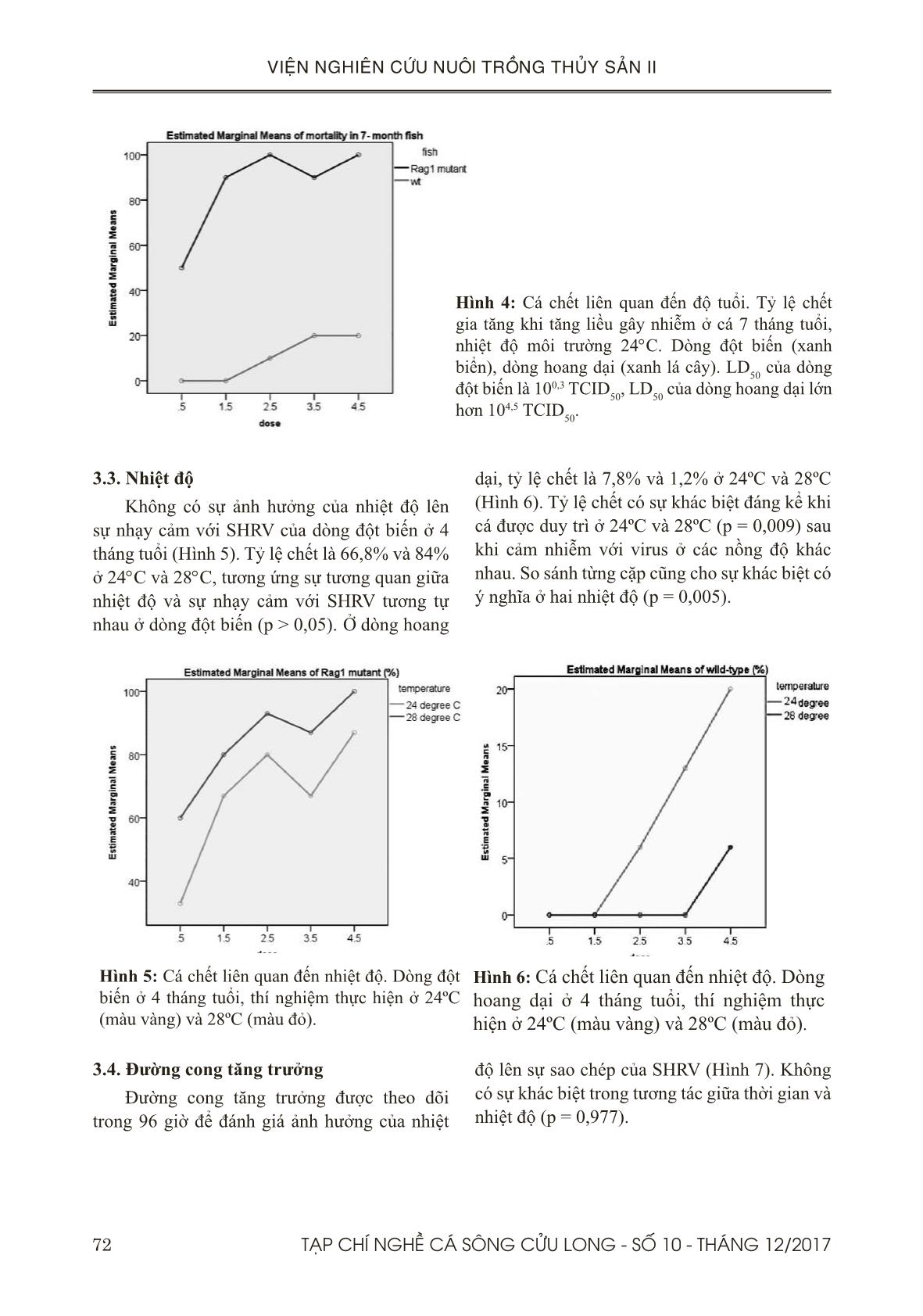
Dòng hoang dại có sự mẫn cảm với virus giảm dần theo độ tuổi. Cá ở 2 tháng tuổi chết nhiều hơn
cá 4 và 7 tháng tuổi khi cho cảm nhiễm với virus. Nhiệt độ cũng gây ảnh hưởng quan trọng trong sự
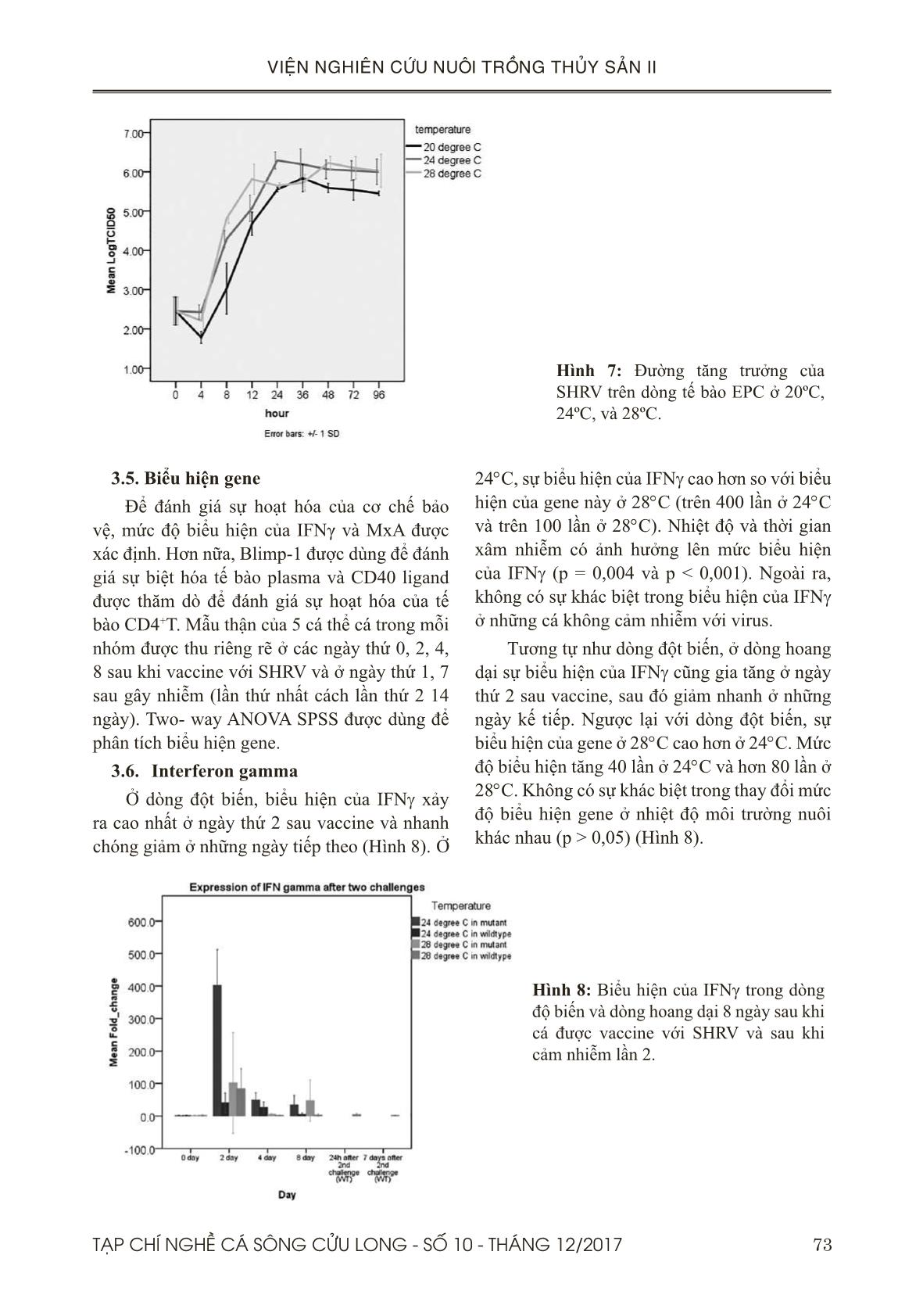
mẫn cảm của chủng hoang dại. Ở 24°C, tỷ lệ cá chết cao hơn ở 28°C. Trong nuôi cấy tế bào, SHRV
có đường cong tăng trường tương tự nhau ở 20°C, 24°C, và 28°C, cho thấy sự tăng sinh của virus
không bị ảnh hưởng ở nhiệt độ thấp. Ngược lại, độ tuổi và nhiệt độ không cho thấy có sự ảnh hưởng
ở chủng đột biến (Rag1 mutant). Tỷ lệ chết cũng khác nhau giữa dòng hoang dại và đột biến. Dòng
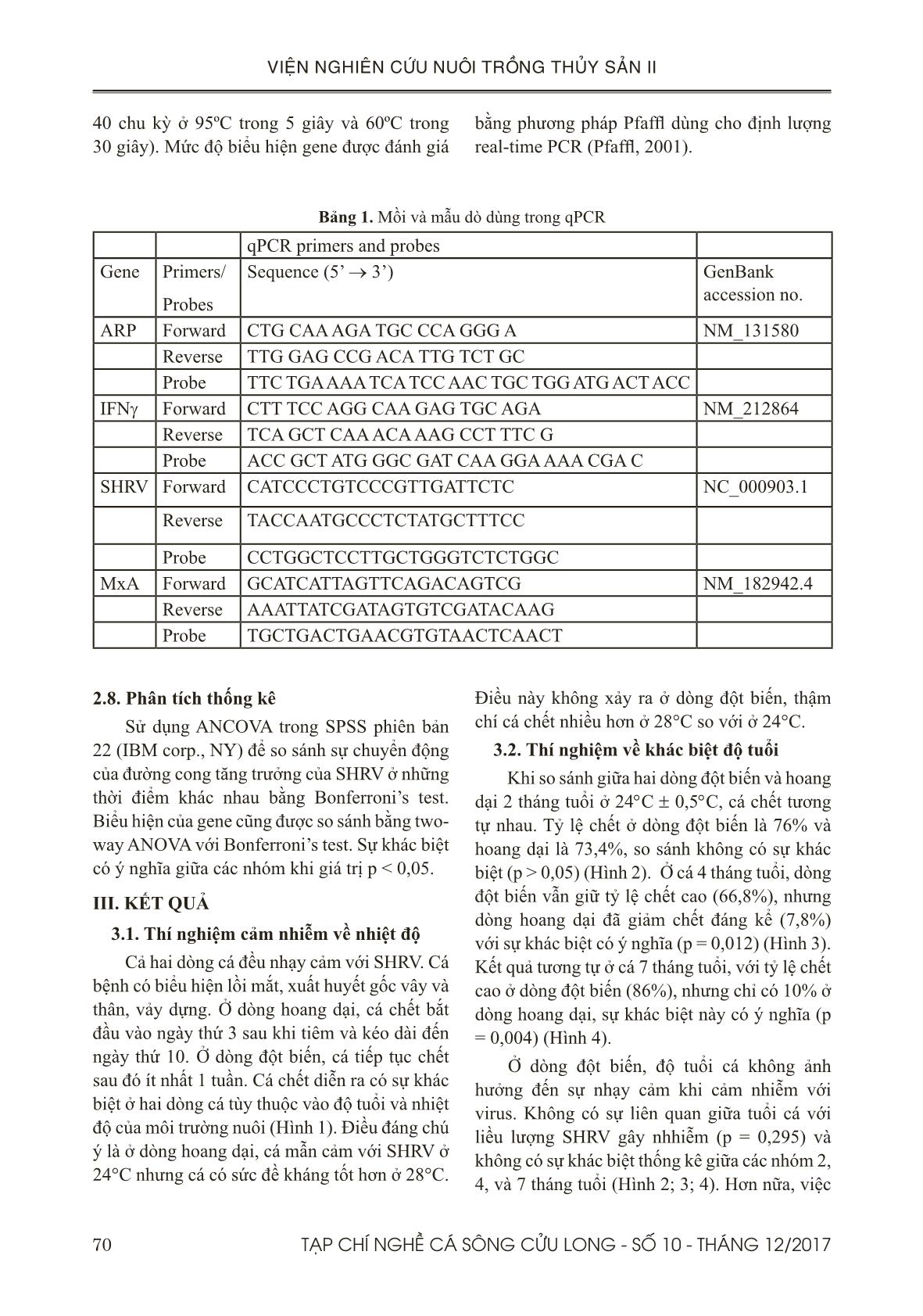
hoang dại bắt đầu chết từ ngày thứ 3 sau khi gây nhiễm và kéo dài đến ngày thứ 10, trong khi dòng
đột biến tiếp tục chết trong vòng một tuần tiếp sau đó.

Trang 1

Trang 2

Trang 3
Trang 4
Trang 5
Trang 6
Trang 7
Trang 8
Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Khác biệt trong sự mẫn cảm với Novirhabdo virus của Zebrafish dòng hoang dại và đột biến và vai trò của lymphocytes

t quả tốt hơn) hoặc thời gian lấy mẫu hơi sớm so với đáp ứng miễn dịch ở cá. TÀI LIỆU THAM KHẢO Ainsworth, A. J., Dexiang, C., Waterstrat, P. R., & Greenway, T. (1991). Effect of temperature on the immune system of channel catfish (Ictalurus punctatus)--I. Leucocyte distribution and phagocyte function in the anterior kidney at 10 degrees C. Comp Biochem Physiol A Comp Physiol, 100(4), 907-912. Alter, G., Malenfant, J. M., Delabre, R. M., Burgett, N. C., Yu, X. G., Lichterfeld, M., . . . Altfeld, M. (2004). Increased natural killer cell activity in viremic HIV-1 infection. J Immunol, 173(8), 5305-5311. Bryceson, Y. T., & Long, E. O. (2008). Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol, 20(3), 344-352. doi: 10.1016/j.coi.2008.03.005 Cancro, M. P., & Smith, S. H. (2003). Peripheral B cell selection and homeostasis. Immunol Res, 27(2-3), 141-148. doi: 10.1385/ir:27:2-3:141 Charoo, Samina Qadir; Salman Rauoof; Qureshi, T.A. (2014). Rainbowtrout (Oncorhynchus mykiss). Journal of Science and Technology, 9(2), 29. Cuchens, Marvin A., & Clem, L. William. (1977). Phylogeny of lymphocyte heterogeneity: II. Differential effects of temperature on fish T-like and B-like cells. Cell Immunol, 34(2), 219-230. doi: 8749(77)90245-3 Degli-Esposti, M. A., & Smyth, M. J. (2005). Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol, 5(2), 112-124. doi: 10.1038/nri1549 Dexiang, C., & Ainsworth, A. J. (1991). Effect of temperature on the immune system of channel catfish (Ictalurus punctatus)--II. Adaptation of anterior kidney phagocytes to 10 degrees C. Comp Biochem Physiol A Comp Physiol, 100(4), 913-918. Doody, G. M., Stephenson, S., & Tooze, R. M. (2006). BLIMP-1 is a target of cellular stress and downstream of the unfolded protein response. Eur J Immunol, 36(6), 1572-1582. doi: 10.1002/ eji.200535646 Fernandez-Trujillo, A., Ferro, P., Garcia-Rosado, E., Infante, C., Alonso, M. C., Bejar, J., . . . Manchado, M. (2008). Poly I:C induces Mx transcription and promotes an antiviral state against sole aquabirnavirus in the flatfish Senegalese sole (Solea senegalensis Kaup). Fish Shellfish Immunol, 24(3), 279-285. doi: 10.1016/j.fsi.2007.11.008 80 TẠP CHÍ NGHỀ CÁ SÔNG CỬU LONG - SỐ 10 - THÁNG 12/2017 VIỆN NGHIÊN CỨU NUÔI TRỒNG THỦY SẢN II Fields, Peter A. (2001). Review: Protein function at thermal extremes: balancing stability and flexibility. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 129(2–3), 417-431. doi: http:// dx.doi.org/10.1016/S1095-6433(00)00359-7 Garcia, J. F., Roncador, G., Garcia, J. F., Sanz, A. I., Maestre, L., Lucas, E., . . . Piris, M. A. (2006). PRDM1/BLIMP-1 expression in multiple B and T-cell lymphoma. Haematologica, 91(4), 467-474. Gong, Y. F., Xiang, L. X., & Shao, J. Z. (2009). CD154- CD40 interactions are essential for thymus- dependent antibody production in zebrafish: insights into the origin of costimulatory pathway in helper T cell-regulated adaptive immunity in early vertebrates. J Immunol, 182(12), 7749- 7762. doi: 10.4049/jimmunol.0804370 Gray, D., Siepmann, K., van Essen, D., Poudrier, J., Wykes, M., Jainandunsing, S., . . . Dullforce, P. (1996). B-T lymphocyte interactions in the generation and survival of memory cells. Immunol Rev, 150, 45-61. Gyory, Ildiko, Wu, Jian, Fejer, Gyorgy, Seto, Edward, & Wright, Kenneth L. (2004). PRDI- BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol, 5(3), 299-308. Haller, O., Arnheiter, H., Lindenmann, J., & Gresser, I. (1980). Host gene influences sensitivity to interferon action selectively for influenza virus. Nature, 283(5748), 660-662. Hilleman, M. R. (2004). Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Natl Acad Sci U S A, 101 Suppl 2, 14560-14566. doi: 10.1073/ pnas.0404758101. Hohn, C., & Petrie-Hanson, L. (2012). Rag1- /- mutant zebrafish demonstrate specific protection following bacterial re-exposure. PLoS One, 7(9), e44451. doi: 10.1371/journal. pone.0044451 Hrubec, T. C., Ward, D., Smith, S. A., & Robertson, J. L. (2004). Age related changes in humoral immune response of hybrid striped bass (Morone chrysops x Morone saxatilis). Vet Immunol Immunopathol, 101(1-2), 103-108. doi: 10.1016/j.vetimm.2004.04.020 Kurata, Osamu, Okamoto, Nobuaki, Suzumura, Eri, Sano, Natsumi, & Ikeda, Yayoi. (1995). Accommodation of carp natural killer-like cells to environmental temperatures. Aquaculture, 129(1–4), 421-424. doi: org/10.1016/0044-8486(94)00282-S Lam, S.H., Chua, H.L., Gong, Z., Lam, T.J., & Sin, Y.M. (2004). Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol, 28, 9-28. Lane, P., Traunecker, A., Hubele, S., Inui, S., Lanzavecchia, A., & Gray, D. (1992). Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol, 22(10), 2573- 2578. doi: 10.1002/eji.1830221016 Le Morvan, C., Troutaud, D., & Deschaux, P. (1998). Differential effects of temperature on specific and nonspecific immune defences in fish. J Exp Biol, 201(Pt 2), 165-168. Lin, K. I., Lin, Y., & Calame, K. (2000). Repression of c-myc is necessary but not sufficient for terminal differentiation of B lymphocytes in vitro. Mol Cell Biol, 20(23), 8684-8695. Magnadóttir, Bergljót, Jónsdóttir, Halla, Helgason, Sigurður, Björnsson, Björn, Jørgensen, Trond Ø, & Pilström, Lars. (1999). Humoral immune parameters in Atlantic cod (Gadus morhua L.): I. The effects of environmental temperature. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 122(2), 173-180. doi: S0305-0491(98)10156-6 Miller, N. W., & Clem, L. W. (1984). Temperature- mediated processes in teleost immunity: differential effects of temperature on catfish in vitro antibody responses to thymus-dependent and thymus-independent antigens. J Immunol, 133(5), 2356-2359. Montoya, C. J., Velilla, P. A., Chougnet, C., Landay, A. L., & Rugeles, M. T. (2006). Increased IFN- gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol, 120(2), 138-146. doi: 10.1016/j.clim.2006.02.008 Noelle, R. J., Roy, M., Shepherd, D. M., Stamenkovic, I., Ledbetter, J. A., & Aruffo, A. (1992). A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A, 89(14), 6550-6554. O’Leary, J, Mahmoud, G, Drayton, D, & Andrian, 81TẠP CHÍ NGHỀ CÁ SÔNG CỬU LONG - SỐ 10 - THÁNG 12/2017 VIỆN NGHIÊN CỨU NUÔI TRỒNG THỦY SẢN II Uv. (2006). T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol, 7, 507 - 516. Page, D. M., Wittamer, V., Bertrand, J. Y., Lewis, K. L., Pratt, D. N., Delgado, N., . . . Traver, D. (2013). An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood, 122(8), e1-11. doi: 10.1182/ blood-2012-12-471029 Paust, S., Senman, B., & von Andrian, U. H. (2010). Adaptive immune responses mediated by natural killer cells. Immunol Rev, 235(1), 286-296. doi: 10.1111/j.0105-2896.2010.00906.x Petrie-Hanson, L., & Ainsworth, A. J. (2001). Ontogeny of channel catfish lymphoid organs. Vet Immunol Immunopathol, 81(1-2), 113-127. Petrie-Hanson, L., & Ainsworth, A.J. (1999). Humoral immune responses of channel catfish (Ictalurus punctatus) fry and fingerlings exposed to Edwardsiella ictaluri.Fish and Shellfish Immunology, 9, 579-589. Petrie-Hanson, L., Hohn, C., & Hanson, L. (2009). Characterization of rag1 mutant zebrafish leukocytes. BMC Immunol, 10(1), 8. doi: 1471- 2172-10-8 [pii] 10.1186/1471-2172-10-8 Petrie-Hanson, Lora, Hohn, Claudia, & Hanson, Larry. (2009). Characterization of rag1 mutant zebrafish leukocytes. BMC Immunology, 10(1), 8. Pfaffl, Michael W. (2001). A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research, 29(9), e45-e45. Reed, L.J., & Muench, H. (1938). A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology, 27(3), 493-497. Reusch, J. A., Nawandar, D. M., Wright, K. L., Kenney, S. C., & Mertz, J. E. (2015). Cellular differentiation regulator BLIMP1 induces Epstein-Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters. J Virol, 89(3), 1731- 1743. doi: 10.1128/jvi.02781-14 Saint-Jean, S. R., & Perez-Prieto, S. I. (2007). Effects of salmonid fish viruses on Mx gene expression and resistance to single or dual viral infections. Fish Shellfish Immunol, 23(2), 390- 400. doi: 10.1016/j.fsi.2006.11.012 Shapiro-Shelef, M., Lin, K. I., McHeyzer-Williams, L. J., Liao, J., McHeyzer-Williams, M. G., & Calame, K. (2003). Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity, 19(4), 607-620. Sharon, N., & Lis, H. (1989). Lectins as cell recognition molecules. Science, 246(4927), 227-234. Somero, George N. (2004). Adaptation of enzymes to temperature: searching for basic “strategies”. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 139(3), 321-333. doi: cbpc.2004.05.003 Son, B. K., Roberts, R. L., Ank, B. J., & Stiehm, E. R. (1996). Effects of anticoagulant, serum, and temperature on the natural killer activity of human peripheral blood mononuclear cells stored overnight. Clin Diagn Lab Immunol, 3(3), 260-264. Sun, J. C., Beilke, J. N., & Lanier, L. L. (2009). Adaptive immune features of natural killer cells. Nature, 457(7229), 557-561. doi: 10.1038/ nature07665 Trede, NS, Langenau, DM, Traver, D, Look, AT, & Zon, LI. (2004). The use of zebrafish to understand immunology. Immunity, 20, 367 - 379. Turner, C. A., Jr., Mack, D. H., & Davis, M. M. (1994). Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell, 77(2), 297-306. van Kooten, C., & Banchereau, J. (2000). CD40- CD40 ligand. J Leukoc Biol, 67(1), 2-17. Vojtech, L. N., Sanders, G. E., Conway, C., Ostland, V., & Hansen, J. D. (2009). Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect Immun, 77(2), 914-925. doi: 10.1128/IAI.01201-08 Wang, R., Jaw, J. J., Stutzman, N. C., Zou, Z., & Sun, P. D. (2012). Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol, 91(2), 299-309. doi: 10.1189/ jlb.0611308 Wienholds, E., Schulte-Merker, S., Walderich, B., & Plasterk, R. H. (2002). Target-selected inactivation of the zebrafish rag1 gene. Science, 297(5578), 99-102. Yoder, J. A., Nielsen, M. E., Amemiya, C. T., & Litman, G. W. (2002). Zebrafish as an immunological model system. Microbes Infect, 4(14), 1469-1478. 82 TẠP CHÍ NGHỀ CÁ SÔNG CỬU LONG - SỐ 10 - THÁNG 12/2017 VIỆN NGHIÊN CỨU NUÔI TRỒNG THỦY SẢN II DIFFERENTIAL SUSCEPTIBILITY OF WILD-TYPE AND T AND B LYMPHOCYTE DEFICIENT ZEBRAFISH INFECTED WITH A NOVIRHABDO VIRUS REVEALS THE ROLE OF LYMPHOCYTES Nguyen Ngoc Du1*, Lorelei Ford2, Lora Petri- Hanson2, Larry Hanson2 ABSTRACT In order to study the contribution of innate defenses and lymphocyte based immunity in protection against a primary viral infection in fish, wild-type and Rag1 mutant zebrafish (T and B lymphocyte deficient) were infected with a novirhabdovirus, Snakehead Rhabdovirus (SHRV), by intraperitoneal (IP) injection at different ages and temperatures. Both strains of fish were highly susceptible to SHRV. Diseased fish demonstrated exophthalmia, hemorrhaged fin bases and protruding scales. The susceptibilities of the wild-type decreased as the fish aged; 2-month post hatch fish had significantly more losses than 4 month old and 7 month old fish. Also, temperature had a significant effect on the susceptibility of the wild-type. The wild-type zebrafish held at 24°C had significantly higher mortality than those held at 28°C. In cell culture, SHRV had similar growth kinetics at 20°C, 24°C, and 28°C suggesting that reduced virus replication was not a factor in the lower mortality. The age and temperature effects were not seen with the Rag1 mutants. There was a difference in mortality pattern between the wild-type and Rag1 mutant. All mortalities in the wild-type occurred between day 3 and 10 post-infection, whereas the Rag1 mutant fish continued to die for at least one more week. To assess differences in the response of NK and T-cells of Rag1 mutant and wild-type zebrafish we evaluated transcript levels of the interferon gamma (IFNγ) gene in the posterior kidney by quantitative reverse transcriptase PCR (qRT-PCR). Expression was low in un-exposed fish but increased substantially in both strains at day 2 after exposure to SHRV. IFNγ over 400-fold and 100-fold in Rag1 mutant at 24°C and 28°C, respectively; and over 40-fold and 80-fold in wild-type at 24°C and 28°C, respectively. The strong IFNγ response in lymphocyte deficient fish suggests Natural Killer cell activation. MxA (Myxovirus Resistance gene A) expression also increased in non- infected zebrafish (at day 8) and in SHRV- infected fish (both strains) at day 2 and day 8. We attempted to evaluate the initial activation of the acquired responses by evaluating of Blimp-1 and CD40 ligand expression at day 8, and at day 1 and day 7 after a second challenge, but no significant induction was detected at this early time point. The infection data suggest that innate immunity is important in controlling virus infection early during the infection, but then the acquired immune system clears the infection. Furthermore, age and the environmental temperature have a strong effect on this acquired immune response. These results may help explain the temperature and age associated resistance observed in other novirhabdovirus diseases in fish. Keywords: Zebrafish, Rag1, T and B lymphocyte deficient, Snakehead Rhabdovirus, interferon gamma, MxA, Blimp-1, CD40 ligand. Người phản biện: TS. Lê Hồng Phước Ngày nhận bài: 11/12/2017 Ngày thông qua phản biện: 25/12/2017 Ngày duyệt đăng: 30/12/2017 1 Southern Monitoring Center for Aquaculture Environment and Epidemic, Research Institute for Aquaculture No.2. 2 Mississippi State University *Email: ngocduaqua@yahoo.com
File đính kèm:
 khac_biet_trong_su_man_cam_voi_novirhabdo_virus_cua_zebrafis.pdf
khac_biet_trong_su_man_cam_voi_novirhabdo_virus_cua_zebrafis.pdf

