Journal of Fisheries science and Technology - No.4/2018
ABSTRACT The white-Striped cleaner shrimp Lysmata amboinensis is a favorite ornamental species in Vietnam and worldwide, but the rearing conditions for larvae of this species has not been studied yet. Therefore, this study was conducted to determine proper conditions for larval rearing of white-striped cleaner shrimp Lysmata amboinensis. The experiment was designed as completely randomized design with 9 treatments, including 3 types of rearing water (disinfected water using chlorine, green-water and biofi lter-water) and 3 types of tank (upwelling, Weis and Kreisel tank). Each treatment had 3 replicates, resulting in a total of 27 experimental units. The experimental units were tanks fi lled with 5L of one of three types of rearing water. The results showed that larval survival was similar among three different water types. Larval survival was higher in Kreisel tanks than in upwelling and Weis tanks. There was no interactive effect between rearing water and tank type on the survival rate of the cleaner shrimp larvae. Therefore, disinfected water (lower operation cost) and Kreisel tank are recommended for rearing of white-striped cleaner shrimps. Keywords: Lysmata amboinensis, white-striped cleaner shrimp, Kreisel, Weis. I. INTRODUCTION
The demand of ornamental organisms has been rising rapidly during the last decades with a total annual value of 200-300 million USD [2; 7]. There are many marine species such as fi nfi sh, starfi sh, jellyfi sh, mollusk and crustacean that are cultured in aquarium nowadays. Among ornamental species, whitestriped cleaner shrimp Lysmata amboinensis is one of the favourite ornamental species as they have attractive appearance and behavior [5]. This species also has high trading value. For example, the price per individual typically varies from 65-85 USD [8]. However, most of them are caught from coral reefs with unsustainable methods, causing high pressure to natural environment [3]. Although Lysmata amboinensis has high market demand and value, there is a lack of studies on the broodstock culture and efforts in rearing larvae are, unfortunately, unsuccessful [8]. Therefore, research on white-striped shrimp production that includes artifi cial seed production is, no doubt, contributing to satisfy local and global market demand

Trang 1

Trang 2
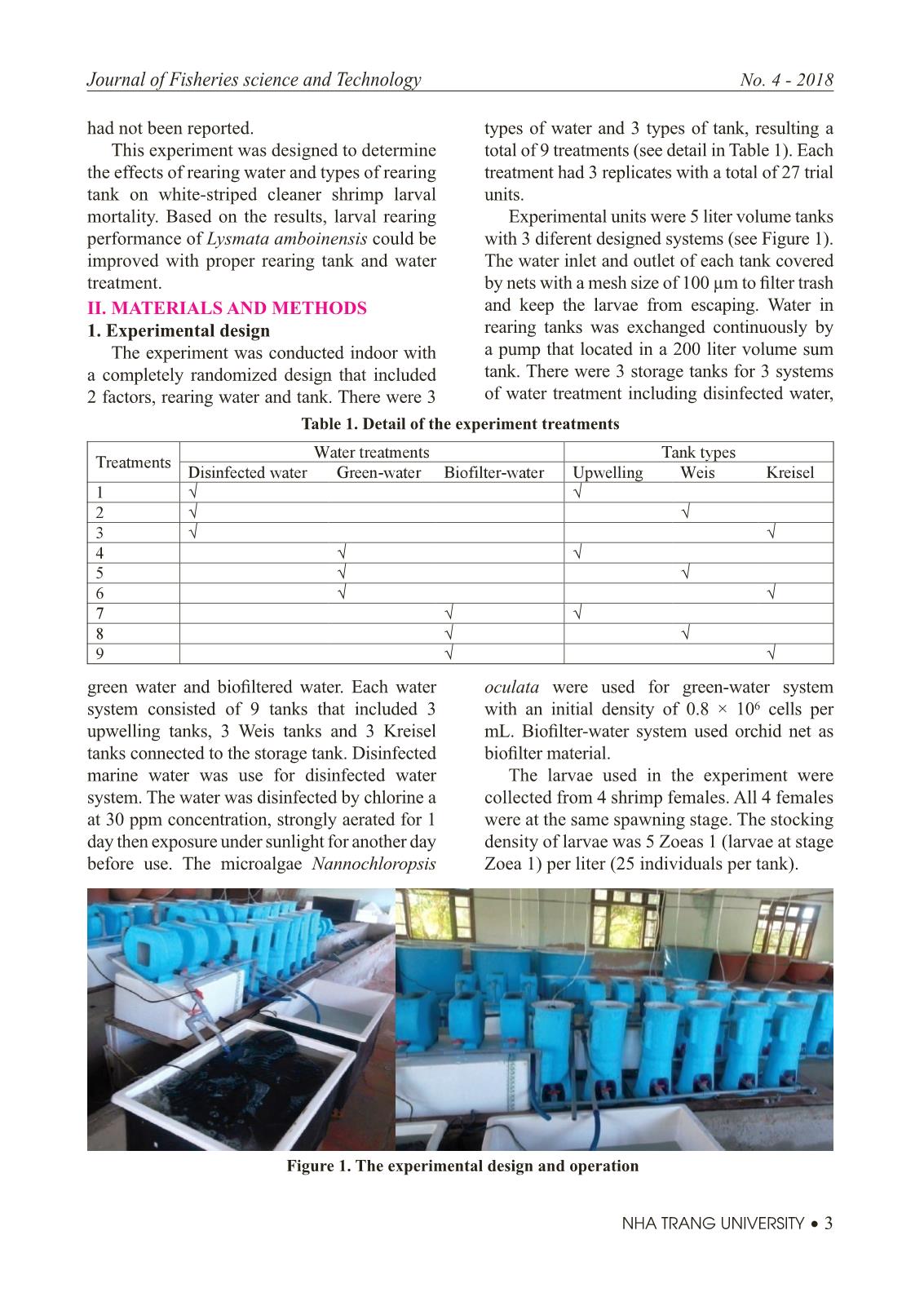
Trang 3

Trang 4
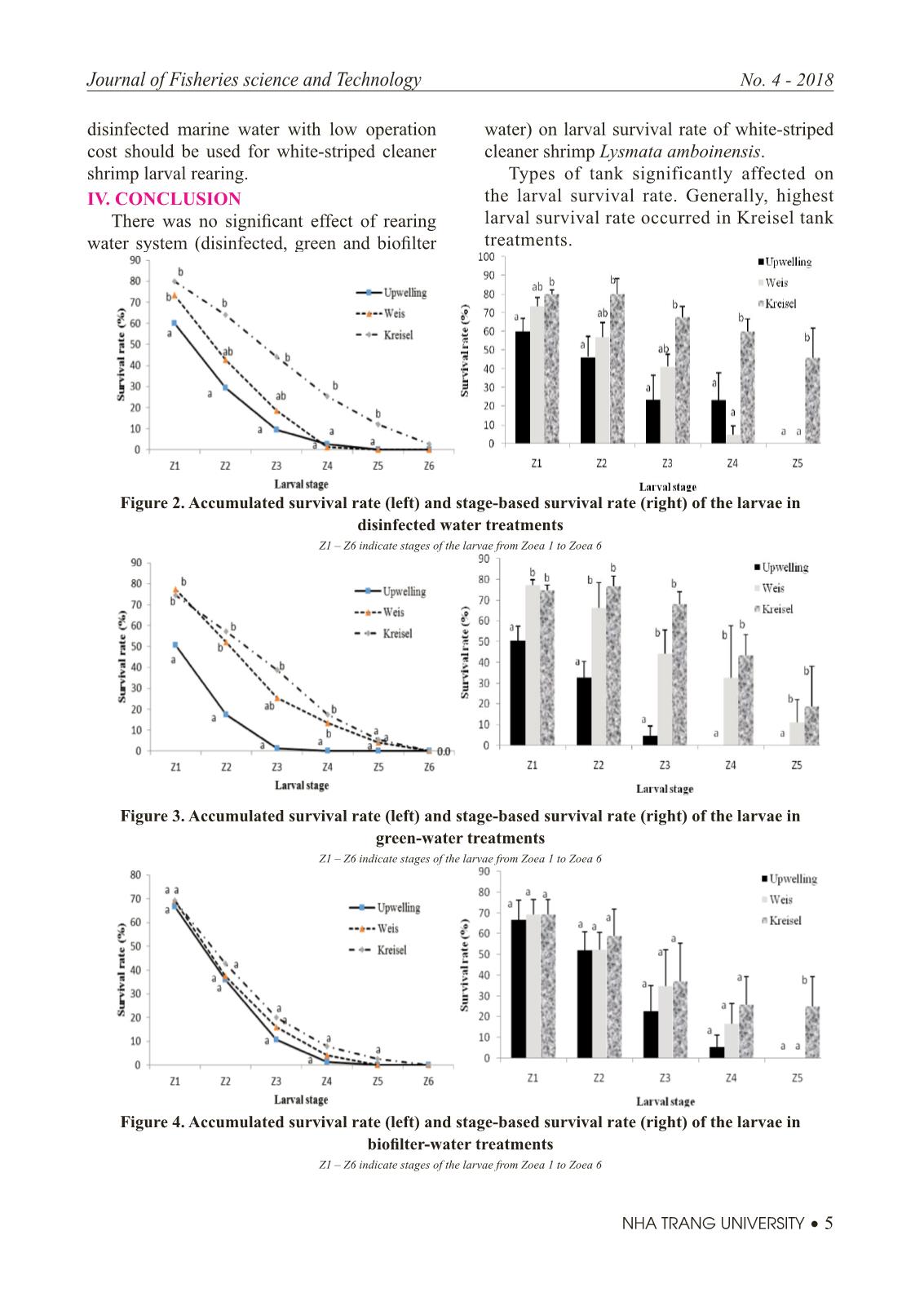
Trang 5

Trang 6

Trang 7
Trang 8
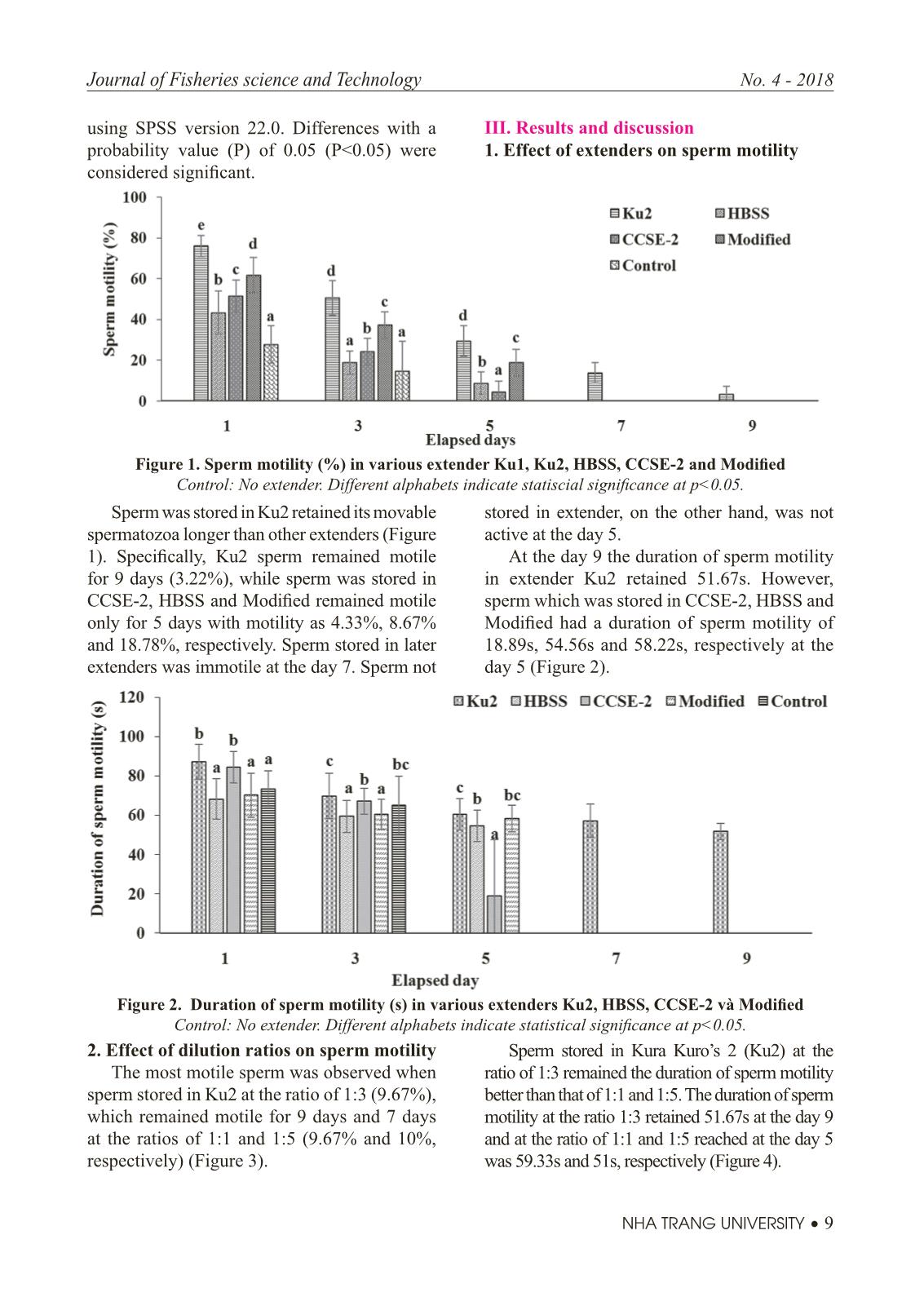
Trang 9
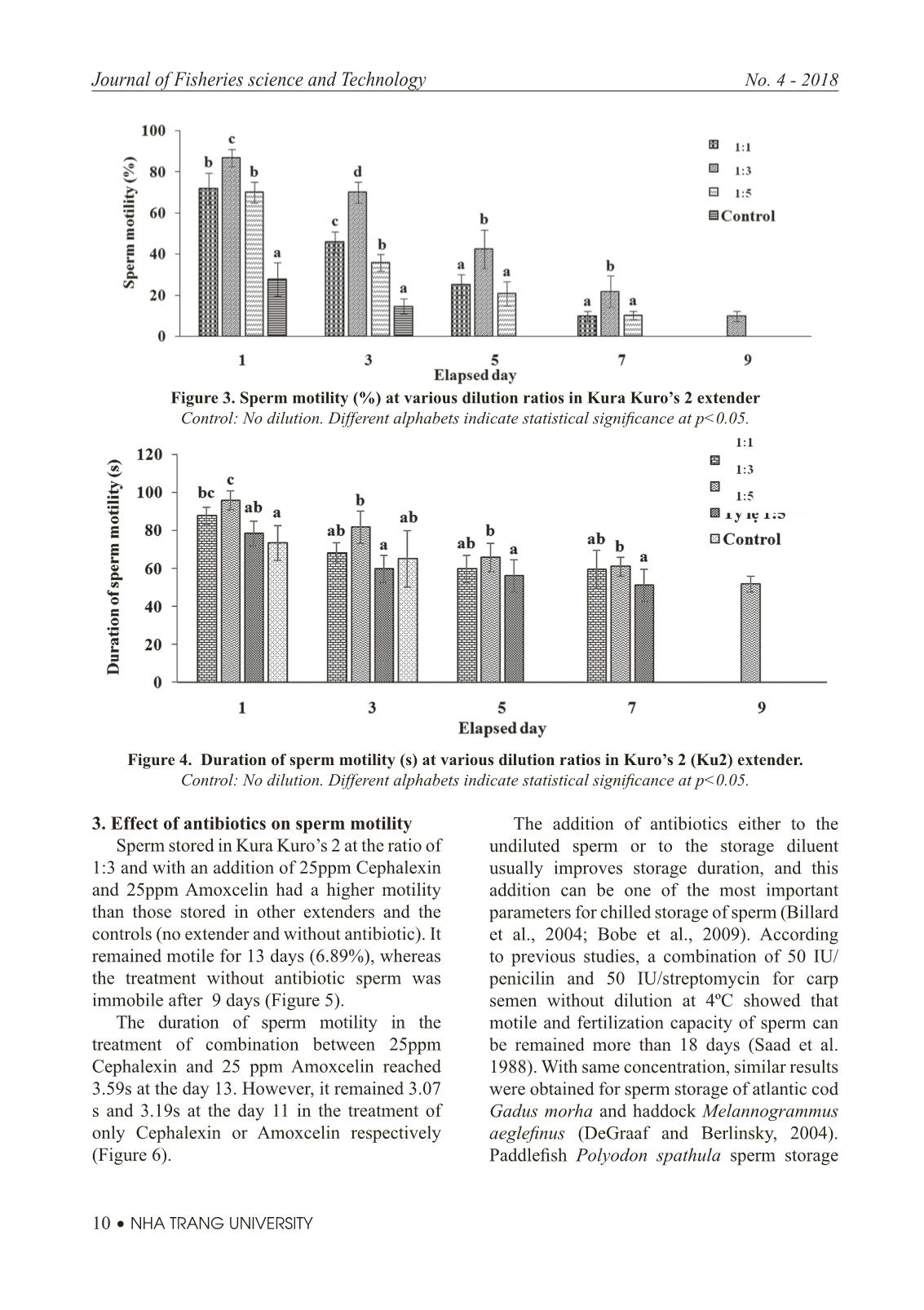
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Journal of Fisheries science and Technology - No.4/2018

reatments, as well as between the MD and the LD treatments (Table 1). Sediment pH was similar among treatments, and relatively stable throughout the experiment. Sediment Chl a concentration was signifi cantly higher in the HD treatment than those in the MD and the LD treatments. Mean value of pore water TAN was signifi cantly higher in the HD treatment than that in the LD treatment. There was no signifi cant difference in mean pore water SRP among treatments (Table 2). The signifi cant differences in some major Table 1: Water parameters in the experimental treatments of rabbitfi sh culture at different stocking densities. Values are means ± SD. Mean values in a same row with different superscript letters are signifi cantly different (P<0.05). Table 2: Sediment parameters in the experimental treatments of rabbitfi sh culture at different stocking densities. Values are means ± SD. Mean values in a same row with different superscript letters are signifi cantly different (P<0.05). environmental parameters between the HD and the LD treatments, (Table 1&2) indicated the effects of rabbitfi sh stocking density on environmental variation in the culture tanks. These effects were possibly derived from the amount of food feeding daily and rabbitfi sh activities. Boyd and Tucker (1998) stated that most of the feed were eaten directly by fi sh, but usually only 10 – 30% of phosphorus (P) and 20 – 40% of nitrogen (N) applied in feed were retained by cultured animals. The remainder of the N and P entered pond ecosystems in faeces or other metabolic products. Depending on the species and culture techniques, up to 85% of P and 52 – 95% of N input into a marine fish culture system as feed might be lost into the environment through feed wastage, fish excretion, faeces production and respiration, and some of 21% of N and 53% of P of feed input accumulated in the bottom sediments (Wu, 1995). N in sediment organic matter may be mineralized to ammonia and recycled to the pond water. P released by decomposition of organic matter in pond bottoms is rapidly adsorbed by sediment and little of it enters the water (Boyd et al., 2002). As the experiment was carried out in the closed tanks, all released waste and nutrients were retained and accumulated in the water columns and sediments over the course of the experiment. The accumulation of waste and nutrients led to increasing and variation of some of the environmental parameters in the culture tanks, especially in the HD treatment. The high 106 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 increases of TAN and SRP in the HD treatment were probably derived from larger quantity of waste, fi sh excretion, nutrients loading from larger amount of feed used in comparison with the lower quantities in the MD and the LD treatments. High concentrations of TAN and SRP might bring about well development of phytoplankton and microphytobenthos in water column and sediment (Table 1&2). In aquaculture ponds, N and P are the two most important nutrients because they are often present in short supply and limit phytoplankton growth (Boyd, 1998). The nutrient concentrations likely increased following the stocking density, and thus got the highest values and wide ranges of variations in the HD treatment (Table 1&2). However, these values still lied in acceptable ranges for ammonia, NH+4 0.2 - 2 mg.L -1 (14.3 – 143.0 µM), NH3< 0.1 mg.L-1 (7.1 µM), and phosphorus, 0.005 – 0.2 mg.L-1 (0.2 – 6.5 µM) in pond aquaculture water (Boyd, 1998). Notably, the present experiment was conducted in a closed system without water exchange, so nutrients released by feed loading and metabolic products would be accumulated within the tanks that probably led to degradation of water quality and then effects on rabbitfi sh growth and survival. 2. Rabbitfi sh growth performance There was no signifi cant difference in rabbitfi sh growth performance among treatments. Fish SR was 100% in the LD and MD treatments, while fi sh mortality strongly occurred in one of replicate of the HD treatment. Rabbitfi sh yield was signifi cantly greater in the MD and the HD treatments than that in the LD treatment, but it was not signifi cantly different between the MD and the HD treatments. Food conversion ration (FCR) was not signifi cantly different between the MD and the LD treatments (Table 3). Table 3: Growth performance of rabbitfi sh cultured at different stocking densities. Mean values in a same row with different superscript letters are signifi cantly different (P<0.05). (*): FCR could not be calculated for the high density treatment because of negative weight gain in a replicate where high mortality occurred. There was no signifi cant difference in rabbitfi sh survival and growth performance among all treatments, indicating that stocking densities at tested levels had no negative effect on rabbitfi sh survival and growth. Similar results were recorded by other authors (Yousif et al. 2005; Saoud et al. 2008). Stocking density may or may not cause adverse effects on fish survival and growth, depending on the species of fish being reared and their development stages (Jorgensen et al. 1993, El-Sayed 2002). Since rabbitfi sh are schooling fish (Lam, 1974) and have tolerance of overcrowding (Ben-Tuvia et al., 1973), little competitive behaviour is expected among individuals reared at high densities. Rabbitfi sh mortality occurred in one of four Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 107 replicates of the HD treatment without known apparent reason. This phenomenon happened near the end of the experimental period when phytoplankton was blooming in the tank as Chl a concentration reached 179.2 µL-1. The toxic gas, such as NH3, was lower than lethal level for fi sh (TAN 0.5 – 1.33 mg.L-1, and NH3 0.02 – 0.09 mg.L-1, which was probably not a reason of rabbitfi sh mortality. But this concentration of ammonia could damage gills and reduce growth of fi sh (Lazur, 2007). An increase in stocking density is desirable since generally reduce production costs per culture area (Huguenin, 1997). However, as biomass increases, so does the quantity of feed offered, resulting in potential eutrophication and oxygen concentration depletion. The results of this study showed that stocking density had no directly negative effect on growth and survival of Siganus lineatus by competing among individuals. High stocking density (in this experiment, 21 fi sh.m-2), however, might cause high environmental variability, as a consequence that adversely affects on fi sh performance. At low density (7 fi sh.m-2), the environment was well maintained, but low yield was produced. Stocking density at 14 fi sh.m-2 seemed to be more suitable for rabbitfi sh rearing in a closed system, produced a relative high yield without widely environmental variations. However, further researches need to be carried out for longer period of culture with different stocking densities at various size groups of rabbitfi sh to determine optimal stocking density and size to optimize high production versus low environmental changes in a closed system. IV. CONCLUSION The results showed that goldlined rabbitfi sh S. lineatus can well adapt and grow in a closed culture system. The fi sh has little competitive behavior among individuals when stocked at size and density of 5.7 g, 7 – 21 fi sh.m-2. The density has no effect on growth performance of S. lineatus, but when increase stocking density from 7 to 14 fi sh.m-2 can elevate harvested yield. The environmental quality can be adversely affected as increasing stocking density (7 – 21 fi sh.m-2), leading to environmental deterioration by potential eutrophication, high water and sediment nutrient concentrations and phytoplankton bloom. The factors associated with hyper - eutrophication could cause fi sh mortality and reduce growth. ACKNOWLEDGEMENTS We are very grateful to the laboratory technical staff at IFREMER, IRD (LAMA) and New Caledonia University for their help in sample analysis. This study was supported by grant from the South Province of New Caledonia and carried out at the IFREMER Saint-Vincent Aquaculture Research Station and the New Caledonia University. I would like to especially thank Pr. Yves Letourneur, Dr. Hugues Lemonnier and Dr. Sebastien Hochard, who provided me many helps to implement the experiment and valuable comments during working. REFERENCES 1. Bariche, M., 2005. Age and growth of Lessepsian rabbitfish from the eastern Mediterranean. Journal of Applied Ichthyology 21, 141 – 145. 2. Borsa, P., Lemer, S., Aurelle, D., 2007. Patterns of lineage diversifi cation in rabbitfi shes. Molecular Phylogenetic and Evolution 44, 427 – 435. 3. Boyd, C.E., 1998. Water quality for pond aquaculture. Department of Fisheries and Alliced Aquacultures Auburn University, Alabama 36849 USA, 37 pp. 4. Boyd, C.E., Tucker, C.S., 1998. Pond Aquaculture Water Quality Management. Kluwer Academic Publishers, Boston, Massachusettes, 700 pp. 5. Boyd, C.E., Wood, C.W., Thunjai, T., 2002. Aquaculture pond bottom soil quality management. Pond Dynamics/ Aquaculture Collaborative Research Support Program Oregon State University, Corvallis, Oregon 97331 – 1641, 41 pp. 108 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 6. Brown, J.W., Chirichetti, P., Crisostomo, D., 1994. A cage culture trial of Siganus randalli in Guam. Asian Fisheries Science 7, 53 – 56. 7. Bwathondi, P.O.J., 1982. Preliminary investigation on rabbitfi sh, Siganus canaliculatus, cultivation in Tanzania. Aquaculture 27, 205 – 210. 8. Duray, M.N., and Southeast Asian Fisheries Development Center, 1998. Biology and culture of siganids. (Rev. ed.). Tigbauan, Iloilo, Philippines: Aquaculture Department, Southeast Asian Fisheries Development Center. 9. El-Sayed, A-F.M., 2002. Effects of stocking density and feeding levels on growth and feed effi ciency of Nile tilapia (Oreochromis niloticus L.) fry. Aquaculture Research 33, 621 –626. 10. Gundermann, N., Popper. D.M., Lichatowich, T., 1983. Biology and life cycle of Siganus vermiculatus (Siganidae, Pisces). Pacifi c Science 37 (2), 165 – 180. 11. Hargreaves, J.A., 1998. Nitrogen biogeochemistry of aquaculture ponds. Aquaculture 166, 181 – 212. 12. Holm-Hansen, O., Lorenzen, C.J., Holms, P.E., Strickland, J.D.H., 1965. Fluorometric determination of chlorophyll. Journal du conseil International pour l’exploitation de la mer 30, 3 – 15. 13. Huguenin, J., 1997. The design, operations and economics of cage culture systems. Aquacultural Engineering 16, 167 – 203. 14. Jaikumar, M., 2012. A review on biology and aquaculture potential of rabbitfi sh in Tamilnadu (Siganus canaliculatus). International Journal of Plant, Animal and Environmental Sciences 2 (2), 57 – 64. 15. Jorgensen, E.H., Christiansen, J.S., Jobling, M., 1993. Effects of stocking density on food intake, growth performance and oxygen consumption in Arctic charr (Salvelinus alpinus). Aquaculture 110, 191 – 204Lam, T.J., 1974. Siganids: their biology and mariculture potential. Aquaculture 3, 325 – 354. 16. Koroleff, F., 1976. Determination of ammonia. In: Methods in Seawater Analysis (ed. by K. Grasshof), pp. 126 – 133. Verlag Chemie Weineim, RFA. 17. Lazur, A., 2007. Growout pond and water quality management. Joint Institute of Food Safety and Applied Nutrition, University of Maryland, 17 pp. 18. Murphy, J., Riley, JP., 1962. A single solution method for the determination of soluble phosphate in sea water. Journal of the Marine Biology Association of the United Kingdom 37, 9 – 14. 19. Nelson, S.G., Lock, S.A., Collins, L.A., 1992. Growth of the rabbitfi sh Siganus randalli Woodland in relation to the feasibility of its culture on Guam. University of Guam Marine Laboratory, Technical report No. 97 20. Parazo, M.M., 1990. Effect of dietary protein and energy level on growth, protein utilization and carcass composition of rabbitfi sh, Sigunus guttatus. Aquaculture 86, 41 – 49. 21. Popper, D., Gundermaun, N., 1975. Some ecological and behavioural aspects of siganid populations in the Red sea and Mediterranean coasts of Israel in relation to their suitability for aquaculture. Aquaculture 6, 127 – 141. 22. Saoud, I.P., Ghanawi, J., Lebbos, N., 2008. Effects of stocking density on the survival, growth, size variation and condition index of juvenile rabbitfi sh Siganus rivulatus. Aquaculture International 16, 109 – 116. 23. SPC, 2008. Rabbitfi sh: A candidate for aquaculture in Pacifi c? Fisheries Newsletter # 127 – October/December 2008. 24. Stephanou, D., Georgiou, G., 2000. Recent experiences on the culture of rabbitfi sh Siganus rivulatus in Cyprus. CIHEAM, Cahiers Options Méditerrannéenes 47, 295 – 301. 25. Von Westernhagen, H., Rosenthal, H., 1976. Some aspects of the suitability of various Philippine siganid species (Siganidae) for mariculture. Aquaculture 9, 297 – 311. 26. Wassef, E.A., Abdul Hady, H.A., 1997. Breeding biology of rabbitfi sh Siganus canaliculatus (Siganidae) in mid Arabian Gulf. Fisheries Research 33, 159 – 166. 27. Wu, R.S.S., 1995. The environmental impact of marine fi sh culture: towards a sustainable future. Marine Pollution Bulletin 31 (4-12), 159 – 166. 28. Yousif, O.M., Kumar, K., Ali, A.A., 2005. Growth performance, feed utilization, survival and body composition of rabbitfi sh Siganus cannaliculatus raised at two different stocking densities in sea net cages. Journal Agriculture Society 17, 14 – 22. Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 109 110 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 111 112 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 113 114 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 115 116 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 117 118 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 119 120 • NHA TRANG UNIVERSITY Journal of Fisheries science and Technology No. 4 - 2018 Journal of Fisheries science and Technology No. 4 - 2018 NHA TRANG UNIVERSITY • 121
File đính kèm:
 journal_of_fisheries_science_and_technology_no_42018.pdf
journal_of_fisheries_science_and_technology_no_42018.pdf

