Preparation of ¹⁸F-NaF radiopharmaceuticals using home-made automatic synthesis module at Hanoi Irradiation Center
The aim of this study is to prepare 18F-NaF radiopharmaceutical using a home-made automatic synthesis module consisting of hardware and software which was made by a researcherteam of Hanoi Irradiation Center, HIC, Hanoi, Vietnam. Fluorine-18 isotopes produced in cyclotron KOTRON13 were transferred to the module and radioactive cation impurities were first removed by cation exchange on a carboxymethyl cation exchange (CM) cartridge, and then 18F- ion were trapped by a quaternary methyl ammonium anion exchange (QMA) cartridge. Finally, 18F- was eluated from the cartridge by isotonic saline water (NaCl 0,9% in water) in the form of 18F-NaF. Time of the preparation process was about 13 minutes. Radiochemical yield of the preparation was as high as 95.5%, in average. The qualities of the product were satisfied the criteria of the United States Pharmacopoeia (USP38). PET/CT bone scaner (skeletal scintigraphy) pre-clinical tests using of the 18 F-NaF product showed good quality of imaging for the entire skeletal and distribution of the 18F-NaF in the kidney and the bladder agreed with it’s natural distribution

Trang 1
Trang 2

Trang 3

Trang 4
Trang 5
Trang 6
Trang 7

Trang 8

Trang 9
Tóm tắt nội dung tài liệu: Preparation of ¹⁸F-NaF radiopharmaceuticals using home-made automatic synthesis module at Hanoi Irradiation Center

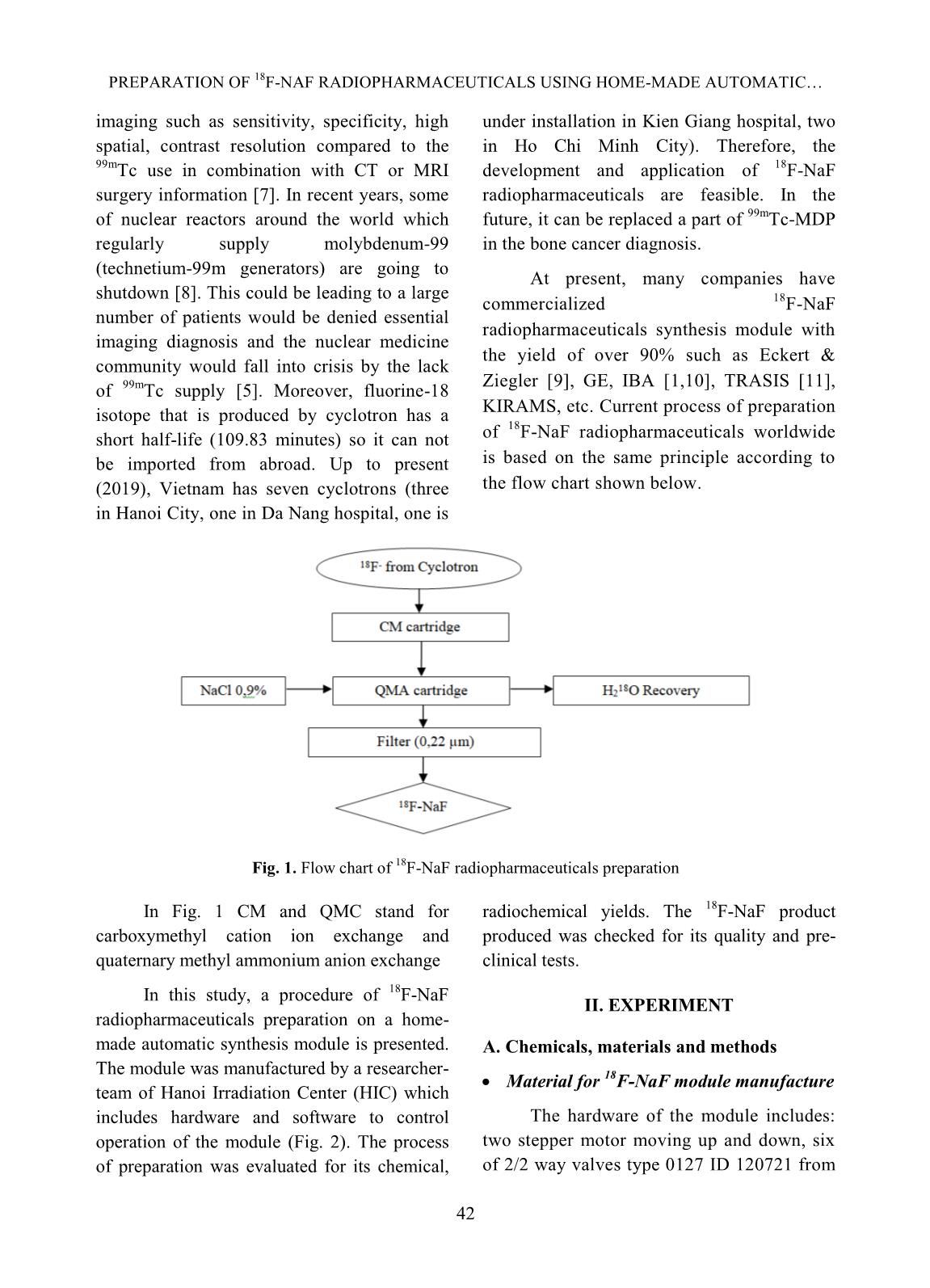
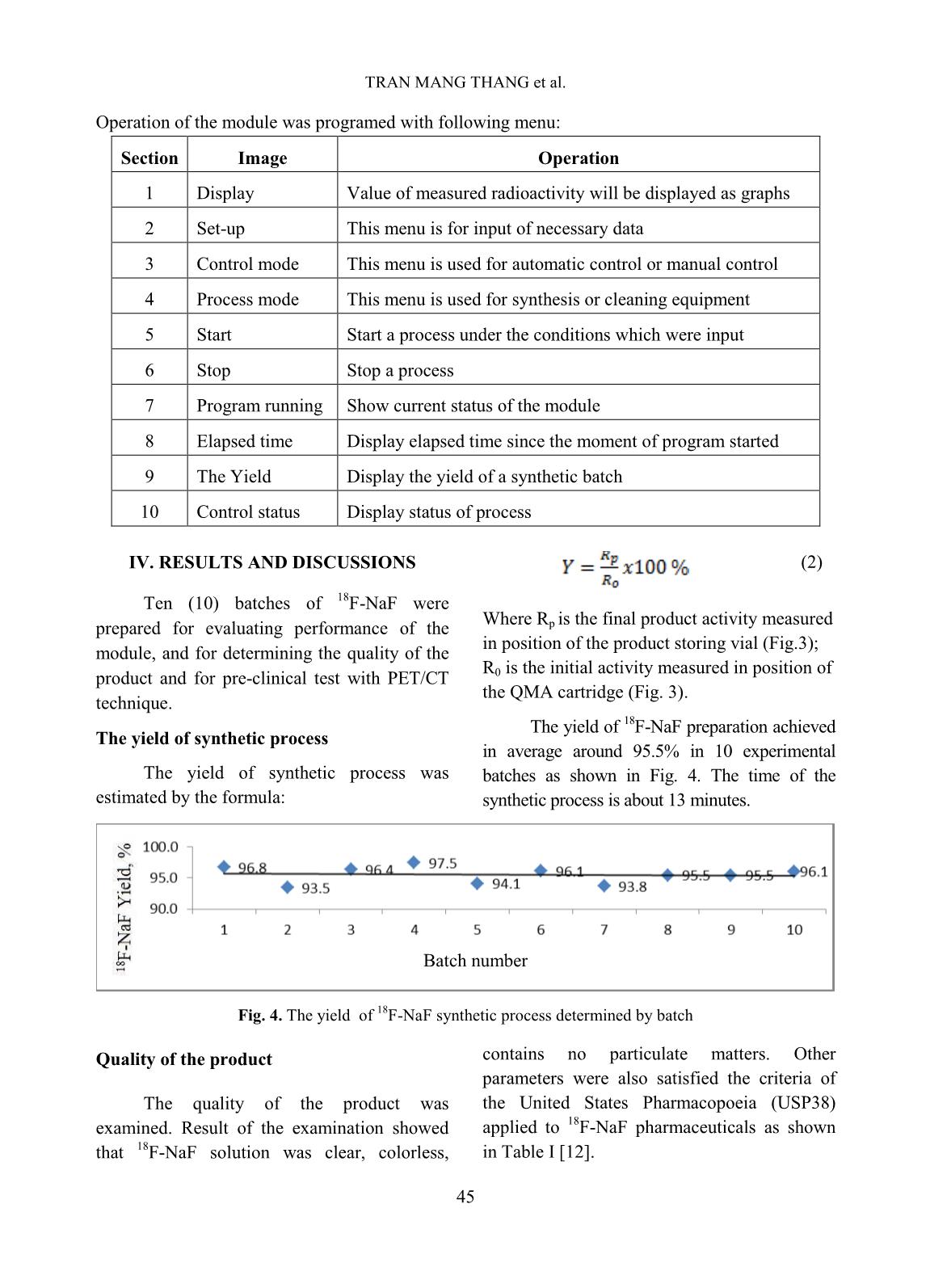
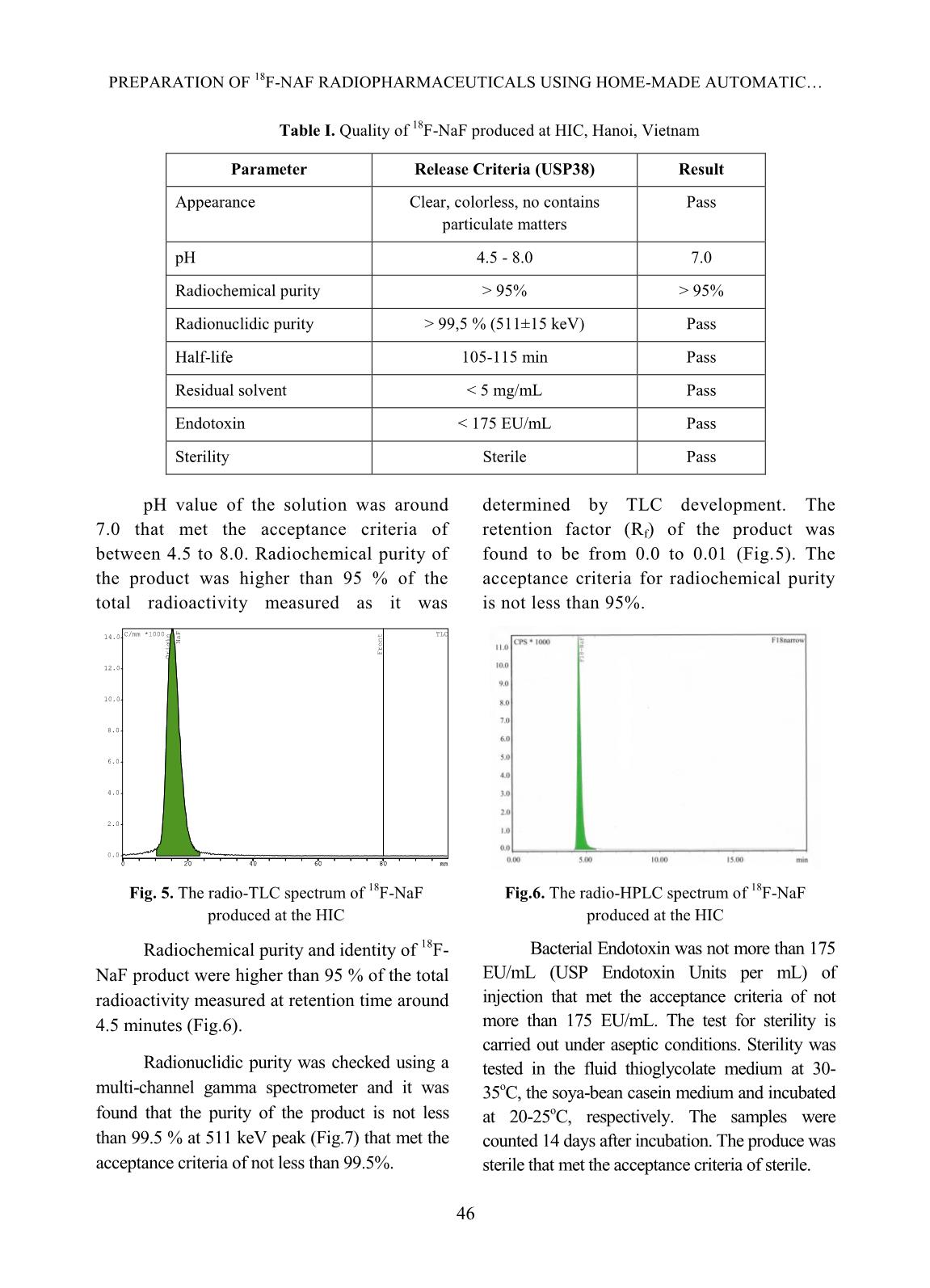
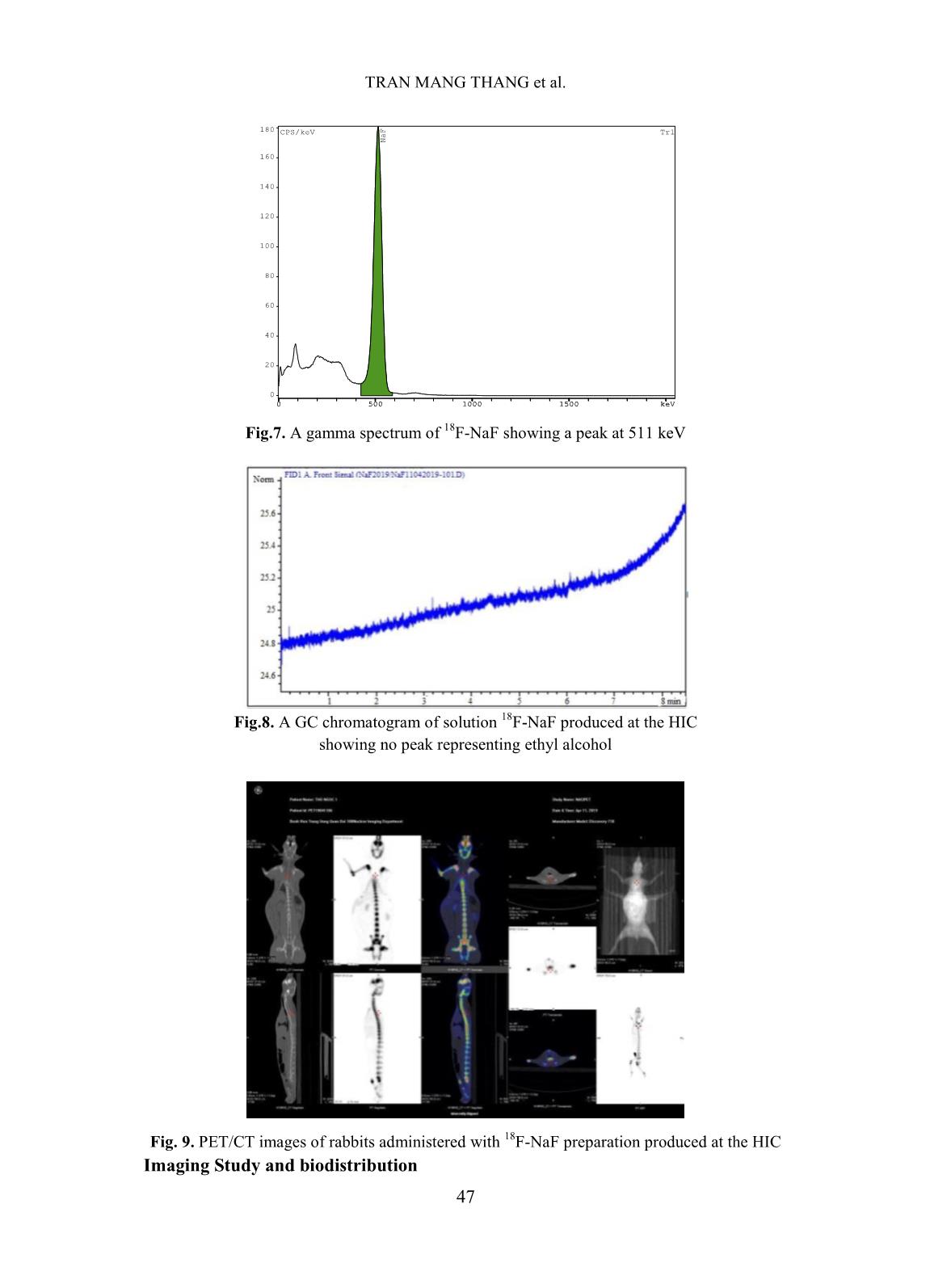
[1,10], TRASIS [11], of 99mTc supply [5]. Moreover, fluorine-18 isotope that is produced by cyclotron has a KIRAMS, etc. Current process of preparation 18 short half-life (109.83 minutes) so it can not of F-NaF radiopharmaceuticals worldwide be imported from abroad. Up to present is based on the same principle according to (2019), Vietnam has seven cyclotrons (three the flow chart shown below. in Hanoi City, one in Da Nang hospital, one is Fig. 1. Flow chart of 18F-NaF radiopharmaceuticals preparation In Fig. 1 CM and QMC stand for radiochemical yields. The 18F-NaF product carboxymethyl cation ion exchange and produced was checked for its quality and pre- quaternary methyl ammonium anion exchange clinical tests. In this study, a procedure of 18F-NaF II. EXPERIMENT radiopharmaceuticals preparation on a home- made automatic synthesis module is presented. A. Chemicals, materials and methods The module was manufactured by a researcher- Material for 18F-NaF module manufacture team of Hanoi Irradiation Center (HIC) which includes hardware and software to control The hardware of the module includes: operation of the module (Fig. 2). The process two stepper motor moving up and down, six of preparation was evaluated for its chemical, of 2/2 way valves type 0127 ID 120721 from 42 TRAN MANG THANG et al. Burkert-Germany, six of 3/2 way valves type and quick-connect Luer adapters from 0127 ID 120433 from Burkert-Germany, two Kinesis-UK, etc. The size of the module is radioactivity monitoring detectors, Teflon 400(H) x 300(L) x 285(W) mm (Fig.2). tube, PEEK and super flangeless fittings, Software for operational control of the flangeless fittings, flangeless fitting for 1/16” module was written on Labview OD tubing, bulk head unions, Y connectors, programming language. Fig. 2. A view of the home-made automatic module used for preparation of 18F-NaF Chemicals and materials for 18F-NaF pH of the preparation was measured synthesis using indicator strips (Macherey-Nagel); 18 18 18 Enriched [ O]-water (H2 O) was half-life of F isotope was determined by a supplied from the Rotem/Israel (18O purity > Dose calibrator (ISOMED2010-Germany); 18 97%). Quaternary methyl ammonium Sep-Pak Chemical purity of F-NaF preparation was Accell Plus Light (QMA) cartridges and analyzed by Radio-TLC development carboxymethyl Sep-Pak Accell Plus Short following by scanning using a Raytest (CM) Cartridges were purchased from the scanner from Germany and Radio-HPLC Waters (USA). Ethyl alcohol (PA grade), Agilent 1260-Raytest; radionuclide purity of 18 acetonitrile (PA grade), glass-based silica thin F-NaF preparation was identified by using layer chromatography (TLC) plates, Millex- multi-channel analyzer (MCA/Raytest- FG filters were from the Merck/Germany. Germany); Residual solvent (ethyl alcohol) AEF filters 0,22µm pore size are from the was determined by Gas chromatography PALL (UK); sterile water for injection and (GC/Agilent 7890B); Bacterial Endotoxins NaCl isotonic saline water (0,9 %) were from was tested by Endotoxin PTS-100 (Charles the Fresenius Kabi Bidiphar/Vietnam. River, USA); Sterility was examined in the Syringes of 20mL capacity were from the BD Dalat Nuclear Research Institute’s (Belgium). Venting needles were from the laboratory. Millipore (USA) and sterile vacuum vials Quality of 18F-NaF radiopharmaceuticals were from Korea. was evaluated at the HIC, Cyclotron Center of Materials and equipment for product’s the 108 Military Central Hospital, and Dalat quality control Nuclear Research Institute laboratories. 43 PREPARATION OF 18F-NAF RADIOPHARMACEUTICALS USING HOME-MADE AUTOMATIC Ossein scintigraphy was performed with 5 mL of enriched 18O-water as a liquid the use of 18F-NaF at a PET/CT Light Speed target was cỉrculated through a Nb cavity device (GE, USA) at Nuclear Medicine covered with Havar foil window. The target Department, 108 Military Central Hospital. was bombarded with proton beam of a current of 30 µA for 60 minutes. Resulting solution Preparation of QMA, CM cartridges containing H18F was transferred into the before its use automatic synthesis module (Fig.2, 3). The Sep-park QMA and CM cartridges need H18F solution first was allowed to pass through to be activated before its use. First, both of the CM cation exchange cartridge that was them were preconditioned with 10 mL ethyl prepared previously to purify all cations alcohol then, they were washed by 12 mL impurities generated from activation reactions sterile water for injection. Finally, they were between protons and the metals made of the dried by blowing a dry air flow. Havar alloy, then the solution was passed 18 - Production of 18F-NaF through QMA cartridge where F anions were 18 radiopharmaceutical trapped on the resin. Finally, F was eluted from the QMA cartridge with 10 mL of 0,9% Fluorine-18 was produced by NaCl isotonic saline solution. The solution of bombarment of protons particles accelerated to 18F-NaF was filtered through an AEF filter of energy 13 MeV on a KOTRON13 cyclotron 0.22 µm pore size into a sterile, pyrogen-free (Korea) located in the HIC. The reaction was vial. Progress of the synthetic processes will be proceeded as follows. shown on screen by an animation scheme (Fig. 18 1 18 1 (1) 3). 8 O 1p 9 F 0 n Fig. 3. Graphical screen showing progress of 18F-NaF preparation process 44 TRAN MANG THANG et al. Operation of the module was programed with following menu: Section Image Operation 1 Display Value of measured radioactivity will be displayed as graphs 2 Set-up This menu is for input of necessary data 3 Control mode This menu is used for automatic control or manual control 4 Process mode This menu is used for synthesis or cleaning equipment 5 Start Start a process under the conditions which were input 6 Stop Stop a process 7 Program running Show current status of the module 8 Elapsed time Display elapsed time since the moment of program started 9 The Yield Display the yield of a synthetic batch 10 Control status Display status of process (2) IV. RESULTS AND DISCUSSIONS Ten (10) batches of 18F-NaF were Where R is the final product activity measured prepared for evaluating performance of the p in position of the product storing vial (Fig.3); module, and for determining the quality of the R is the initial activity measured in position of product and for pre-clinical test with PET/CT 0 the QMA cartridge (Fig. 3). technique. The yield of 18F-NaF preparation achieved The yield of synthetic process in average around 95.5% in 10 experimental The yield of synthetic process was batches as shown in Fig. 4. The time of the estimated by the formula: synthetic process is about 13 minutes. Batch number Fig. 4. The yield of 18F-NaF synthetic process determined by batch Quality of the product contains no particulate matters. Other parameters were also satisfied the criteria of The quality of the product was the United States Pharmacopoeia (USP38) examined. Result of the examination showed applied to 18F-NaF pharmaceuticals as shown that 18F-NaF solution was clear, colorless, in Table I [12]. 45 PREPARATION OF 18F-NAF RADIOPHARMACEUTICALS USING HOME-MADE AUTOMATIC Table I. Quality of 18F-NaF produced at HIC, Hanoi, Vietnam Parameter Release Criteria (USP38) Result Appearance Clear, colorless, no contains Pass particulate matters pH 4.5 - 8.0 7.0 Radiochemical purity > 95% > 95% Radionuclidic purity > 99,5 % (511±15 keV) Pass Half-life 105-115 min Pass Residual solvent < 5 mg/mL Pass Endotoxin < 175 EU/mL Pass Sterility Sterile Pass pH value of the solution was around determined by TLC development. The 7.0 that met the acceptance criteria of retention factor (Rf) of the product was between 4.5 to 8.0. Radiochemical purity of found to be from 0.0 to 0.01 (Fig.5). The the product was higher than 95 % of the acceptance criteria for radiochemical purity total radioactivity measured as it was is not less than 95%. 14.0 C/mm *1000 TLC NaF Front Origin 12.0 10.0 8.0 6.0 4.0 2.0 0.0 0 20 40 60 80 mm Fig. 5. The radio-TLC spectrum of 18F-NaF Fig.6. The radio-HPLC spectrum of 18F-NaF produced at the HIC produced at the HIC Radiochemical purity and identity of 18F- Bacterial Endotoxin was not more than 175 NaF product were higher than 95 % of the total EU/mL (USP Endotoxin Units per mL) of radioactivity measured at retention time around injection that met the acceptance criteria of not 4.5 minutes (Fig.6). more than 175 EU/mL. The test for sterility is carried out under aseptic conditions. Sterility was Radionuclidic purity was checked using a tested in the fluid thioglycolate medium at 30- multi-channel gamma spectrometer and it was 35oC, the soya-bean casein medium and incubated found that the purity of the product is not less at 20-25oC, respectively. The samples were than 99.5 % at 511 keV peak (Fig.7) that met the counted 14 days after incubation. The produce was acceptance criteria of not less than 99.5%. sterile that met the acceptance criteria of sterile. 46 TRAN MANG THANG et al. 180 CPS/keV Tr1 NaF 160 140 120 100 80 60 40 20 0 0 500 1000 1500 keV Fig.7. A gamma spectrum of 18F-NaF showing a peak at 511 keV Fig.8. A GC chromatogram of solution 18F-NaF produced at the HIC showing no peak representing ethyl alcohol Fig. 9. PET/CT images of rabbits administered with 18F-NaF preparation produced at the HIC Imaging Study and biodistribution 47 PREPARATION OF 18F-NAF RADIOPHARMACEUTICALS USING HOME-MADE AUTOMATIC Pre-clinical study is carried out at Institute is acknowledged. The authors would Nuclear Medicine Department, 108 Military like to thank Dr. Jung Young Kim, Korea Central Hospital in Hanoi. Subject of the Institute of Radiological & Medical Sciences test were rabbits to which were injected for his valuable discussions during the study. about 0.3-0.4 mCi (about 0.14 mCi/kg) of 18F-NaF solution. The uptake time for REFERENCE animals was 40 minutes. Afterwards, [1]. Natalia M. Leonardi, Guillermo A. Casale, animals were scaned with a low-dose CT, Jorge Nicolini, Patricia D. Zubata, and Marıa J. full body PET scanner. PET attenuation Salgueiro. Validation of a paper images were corrected by CT. Standardized chromatographic methodology as an uptake values (SUV) are the maximum alternative for determination of the values at the region of interest and radiochemical purity of Na18F, Nucl. Med. calculated in g/mL. Technol., 40: 271-274, 2012. Images of 18F-NaF showed that [2]. Yuan H., Merrill J. R., Parrott M. and 18 metabolism of the radiopharmaceuticals spread Anzellotti A. [ F]NaF Manufacture via the ABT BG75 system. Imaging and throughout the entire skeletal of the animal: in Biodistribution studies.” University of North the skull bone, ribs bones, spine bones, upper Carolina at Chapel Hill, NC27599, School of extremities, two lower limbs, and pelvis. Medicine, pp.1-6. Distribution of 18F-NaF product in kidney and [3]. Mark S. Jacobson, Raymond A. Steichen, and bladder shows it’s natural distribution. Patrick J. Peller. PET radiochemistry and radiopharmacy", Springer-Verlag Berlin III. CONCLUSIONS Heidelberg, 703: 19-30, 2012. In this study, it was proven that the Hanoi [4]. Rajeev Kumar, Rajendra G. Sonkawade, Irradiation Center has successfully manufactured Madhavi Tripathi, Punit Sharma, Priyanka automatic synthesis module for 18F-NaF Gupta, Praveen Kumar, Anil K. Pandey, radiopharmaceuticals. The duration of the Chandrasekhar Bal, Nishikant Avinash Damle, and Gurupad Bandopadhayaya. Production of synthetic process was about 13 mins. the PET bone agent 18F-fluoride ion, Radiochemical yield was as high as 95.5%, in simultaneously with 18F-FDG by single run of average. Quality of the product satisfies the criteria the medical cyclotron with minimal radiation of the United States Pharmacopoeia (USP38). The exposure - A novel technique, Hell J. Nucl. PET/CT imaging test on animals showed good Med., 17: 106-110, 2014. quality for entire skeletal, kidney and bladder. [5]. Brian G. Hockley and Peterj H. Scott. An automated method for preparation of [18F] ACKNOWLEDGMENTS sodium fluoride for injection, USP to address the technetium-99m isotope shortage. Appl. The financial support to conduct this Radiat. Isot., 68: 117-119, 2010. research was from Ministry of Science and Technology of Vietnam under Project encoded [6]. Jae Yong Choi, Ji Woong Lee, Kyo Chul Lee, ĐTCB.07/16/TTCX. The authors are thankful Kyeong Min Kim, Young beom Seo, Jung Young Kim, Young Hoon Ryu. Time and to Vietnam Atomic Energy Institute, and Hanoi cost effective production of sodium Irradiation Center for the administrative [18F]fluoride using a dedicated automation assistances. The co-operation of 108 Military module with disposable cassettes for GMP Central Hospital and Dalat Nuclear Research 48 TRAN MANG THANG et al. environment. J. Radioanal. Nucl. Chem., 309: [10]. Bernard Lambert, Jean-Jacques Cavelier, 938-987, 2016. Guillaume Gauron, Christophe Sauvage, [7]. Frederick D. Grant, Frederic H. Fahey, Alan B. Cécile Kech, Tim Neal, M. Kiselev,David Packard, Royal T. Davis, Abass Alavi, and S. Caron, Anat Shirvan, Ilan Ziv. Routine 18 Ted Treves. Skeletal PET with 18F-Fluoride: automated production of F-labelled Applying new technology to an old tracer. The radiopharmaceuticals on IBA Synthera® th J. of Nucl. Med., 49(1): 68-78, 2008. Multi-Purpose Platform. Presented at the 13 WTTC 2010 in RISOE/Denmark., Abstract [8]. Nuclear and Radiation Studies Board Division No.016, 2010. on Earth and Life Studies. Opportunities and approaches for supplying molybdenum-99 and [11]. CharlotteCollet, Muhammad Otabashi, associated medical isotopes to Global Markets. Fabrice Giacomelli ,Nicolas Veran, Gilles In Proc of a Sym. Washington, DC. The Karcher, Yves Chapleur, Sandrine Lamandé- National Academies Press. Chapter 3: Current Langle. Fully automated production of sodium molybdenum-99 supply, pp. 13-17, 2018. [18F]fluoride on All In One and mini All In [9]. Modular-Lab PharmTracer, Cassette-based One synthesizers. Appl. Radiat.and Isot., 102: routine production of 18F tracers, Eckert & 87-92, 2015. Ziegler, https://radiopharma.com/wp- [12]. The United States Phamacopeial Convention, content/uploads/2018/01/7131-0077.pdf (7131-0077/Rev.04/05. 2019). Accessed on 19 “Sodium FluorideF-18 Injection”, USP38, pp. November 2019. 3546-3547, 2015. 49
File đính kèm:
 preparation_of_f_naf_radiopharmaceuticals_using_home_made_au.pdf
preparation_of_f_naf_radiopharmaceuticals_using_home_made_au.pdf

