Study on the propagation of Actinidia latifolia and actinidia deliciosa in Lam Dong province, Vietnam
Actinidia latifolia and Actinidia deliciosa (kiwifruit) are two species with many applications
in food and medicine. They are capable of growing and developing in Lam Dong province
and similar temperate climates. This study assesses the effect of three plant growth regulators
(NAA, IBA, and IAA) on the root formation of Actinidia latifolia cuttings. At the same time,
seed germination and the effect of various soil and coir mixtures on the growth of kiwifruit
seedlings were tested in the nursery. The results for the Actinidia latifolia cuttings after 60
days showed that NAA and IBA at 1.00% concentration gave the best results, with a rooting
percentage of 76.67%, number of roots/cutting of 3.91, and length of roots/cutting of 5.65 cm
for NAA. For IBA at 1.00% concentration, the rooting percentage was 66.67%, the number
of roots/cutting was 2.43, and the length of roots/cutting was 4.42 cm. When using IAA, the
concentration of 1.50% brought the best results, with a rooting percentage of 66.67%,
number of roots/cutting of 2.81, and length of roots/cutting of 4.34 cm. The germination
percentage of kiwifruit reached 81.00% after 25 days. The best growth of Actinidia deliciosa
seedlings was in a media mixture of 25.00% soil and 75.00% coconut coir, with survival
percentage, height of seedlings, and number of leaves/seedling of 96.00%, 5.02 cm, and 7.17
leaves, respectively, after 45 days.

Trang 1

Trang 2

Trang 3

Trang 4

Trang 5
Trang 6
Trang 7
Trang 8

Trang 9

Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Study on the propagation of Actinidia latifolia and actinidia deliciosa in Lam Dong province, Vietnam

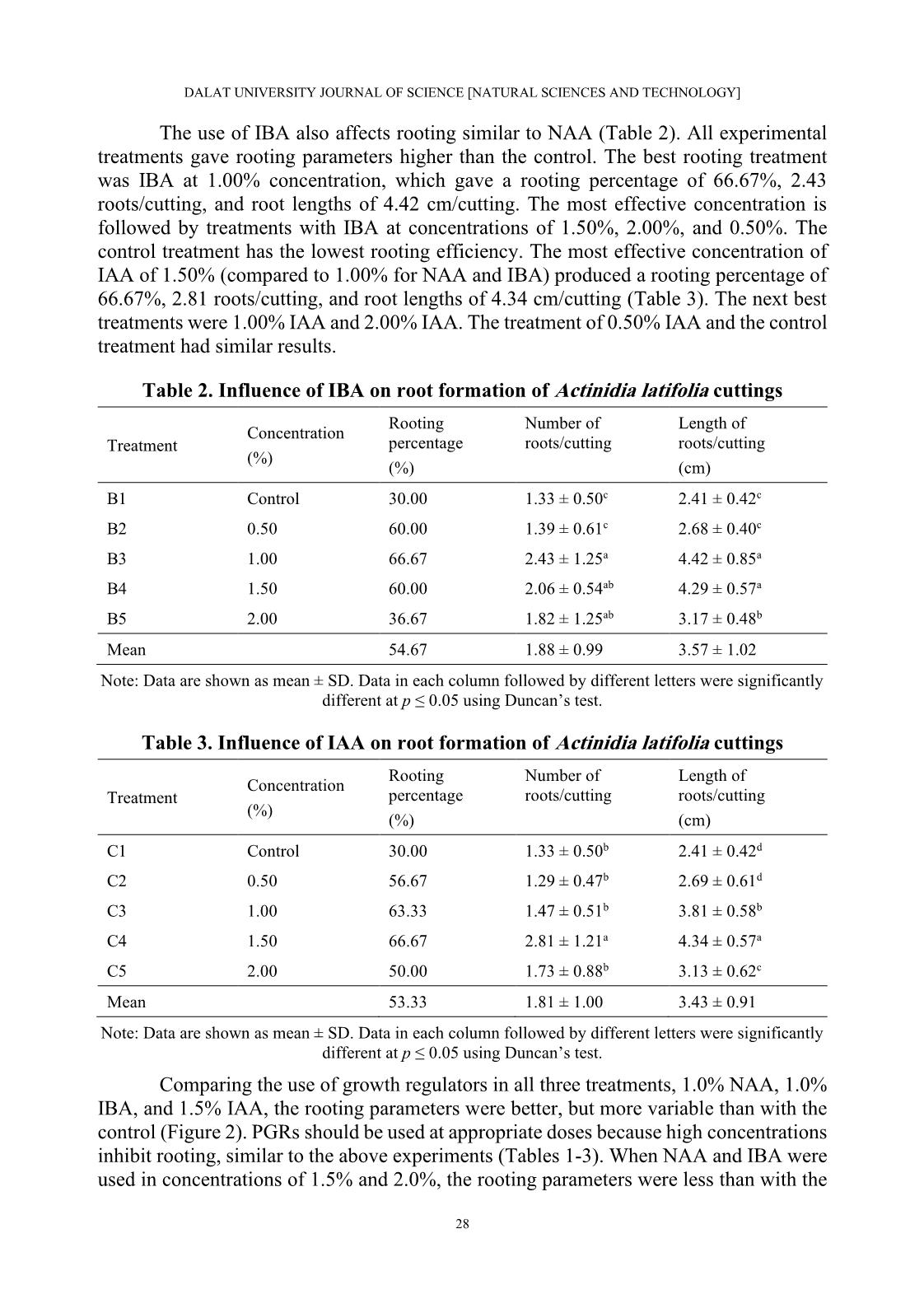
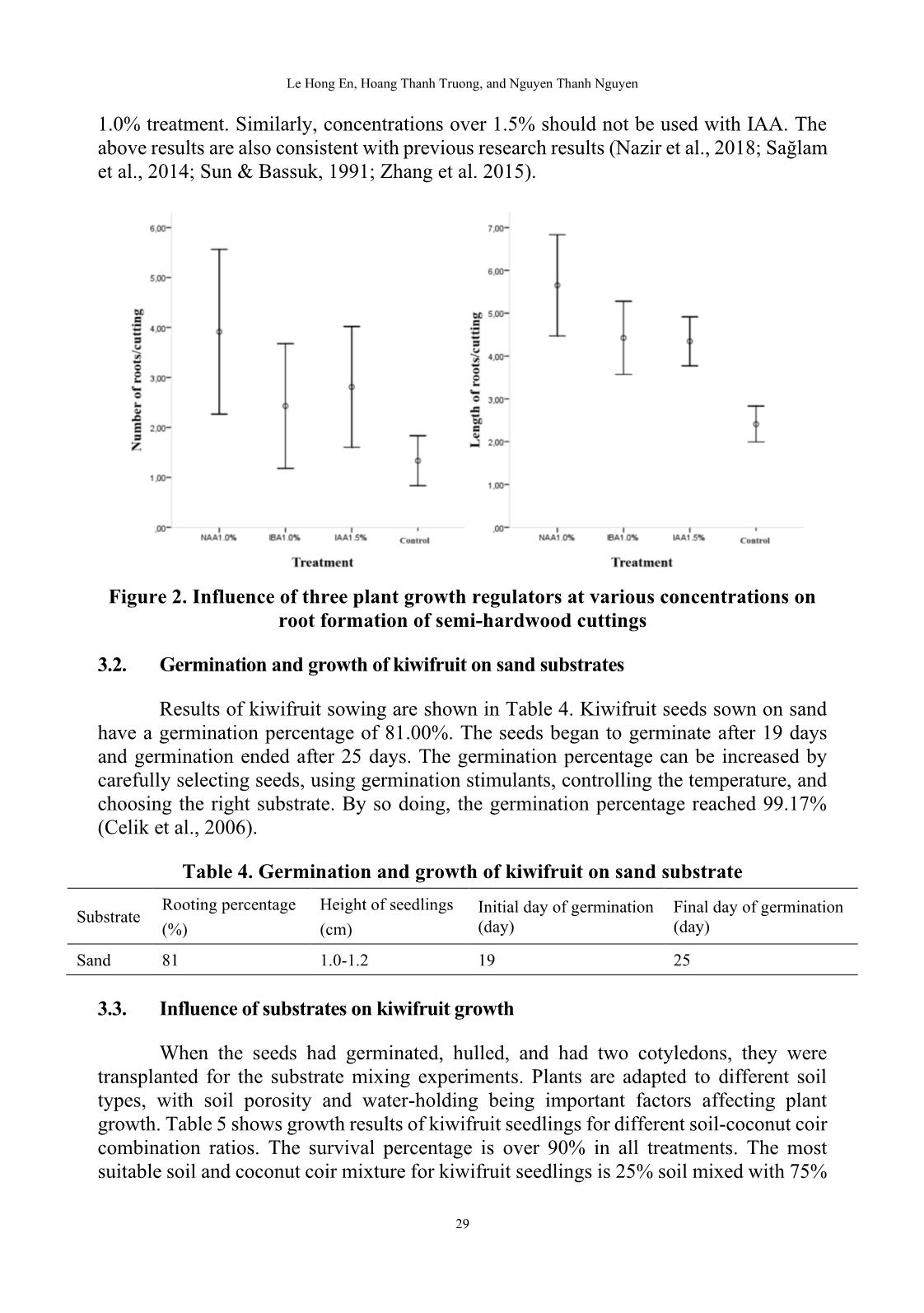
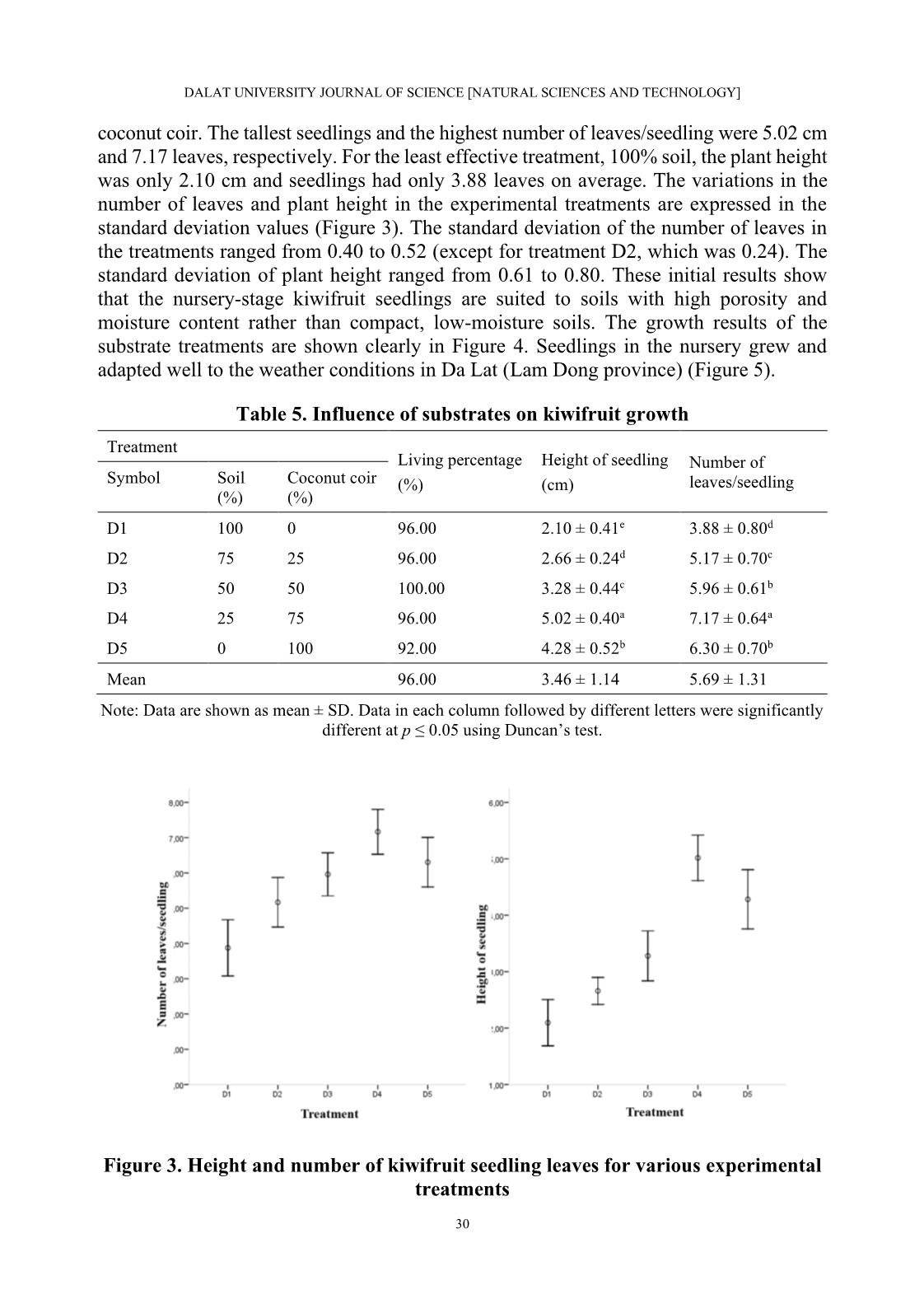
1, B2, B3, B4, and B5, respectively). The experiment was performed on a sand tray in a greenhouse. Each treatment used 30 cuttings that were harvested after 60 days. The rooting percentage (%), number of roots/cutting, and length of roots/cutting (cm) were recorded. • Influence of IBA on root formation of Actinidia latifolia cuttings: The experimental material is Actinidia latifolia semi-hardwood cuttings of about 10-12 cm in length (Figure 1). IBA plant growth regulator was used in concentrations of 0.0%, 0.5%, 1.0%, 1.5%, and 2.0% (denoted C1, C2, C3, C4, and C5, respectively). The experiment was performed on a sand tray in a greenhouse. Each treatment used 30 cuttings that were harvested after 60 days. The rooting percentage (%), number of roots/cutting, and length of roots/cutting (cm) were recorded. 25 DALAT UNIVERSITY JOURNAL OF SCIENCE [NATURAL SCIENCES AND TECHNOLOGY] • Germination and growth of kiwifruit on sand substrates: The materials are green kiwifruit seeds extracted from fresh fruit and washed under tap water. The experiment was performed on a sand tray in a greenhouse. The treatment used 100 seeds. The plants were harvested after 30 days and the following data were recorded: germination percentage (%), height of seedlings (cm), the initial day of germination (day), and the final day of germination (day). • Influence of different growth media mixtures on kiwifruit seedling growth: The experimental materials were kiwifruit seedlings with a height of 1.0-1.2 cm. The substrate test was based on the mixing ratio of coconut coir and soil with coir contents of 0%, 25%, 50%, 75%, and 100% (denoted D1, D2, D3, D4, and D5, respectively). The experiment was performed in plastic bags of size 6 x 12 cm placed in the greenhouse. Each treatment used 25 plants that were harvested after 60 days, and the survival percentage (%), height of seedlings (cm), and number of leaves/seedling were recorded. 2.3. Statistical analysis The statistical analysis was performed with Statistical Package for Social Sciences (SPSS) software version 16.0 using Duncan’s range test (Duncan, 1955). 3. RESULTS AND DISCUSSION 3.1. Influence of NAA, IBA, and IAA on root formation of Actinidia latifolia cuttings Phytohormones are auxiliary substances that act as plant growth regulators (PGRs) under nursery conditions to increase the number of seedlings, shorten rooting time, increase rooting percentage, and increase the number of roots per plant (Kulevanona, 2011). Many research results show that PGRs influence rooting parameters and that different types of PGRs are suitable for different plant species. Nazir et al. (2018) showed that Taxus wallichiana cuttings are best rooted using 1,000 ppm IBA and cuttings in the spring (March to May). Salvia fruticosa has the best rooting results when IAA is used (Sağlam et al., 2014). Zhang et al. (2015) compared the effects of NAA, IBA, and IAA on Carya illinoinensis and demonstrated that the rooting effect on this plant is best with NAA, intermediate with IBA, and least with IAA. Results of experiments that evaluated three different types of PGRs (NAA, IBA, and IAA) on Actinidia latifolia rooting formation to find suitable concentrations and PGRs for cuttings are shown in Table 1. The rooting percentage in the treatments with NAA at all concentrations were higher than for the control. The rooting percentages for the NAA treatments ranged from 50.00% to 76.67%, compared to the rooting percentage of 30.00% for the control. The rooting percentage was highest with the 1.00% NAA treatment (2.5 times higher than the control). In addition to the rooting percentage, the number of roots and their lengths are very important in assessing the quality of the 26 Le Hong En, Hoang Thanh Truong, and Nguyen Thanh Nguyen cuttings. The number of roots was lowest in the control treatment (1.33 roots/cutting) and highest in the 1.00% NAA treatment (3.91 roots/cutting). The root lengths were in the range of 2.41-5.65 cm/cutting with the shortest lengths for the control treatment and the longest for the 1.00% NAA treatment. For an experiment using NAA, a concentration of 1.00% is recommended for the best cuttings results. Figure 1. Experimental Actinidia latifolia cuttings Note: (a) Branch with flowers; (b, c) Branches with fruit; (d) Cuttings (left to right: softwood, semi- hardwood, and hardwood); (e) Callus formation cuttings and rooting cuttings. Table 1. Influence of NAA on root formation of Actinidia latifolia cuttings Rooting Number of Length of Concentration Treatment percentage roots/cutting roots/cutting (%) (%) (cm) A1 Control 30.00 1.33 ± 0.50c 2.41 ± 0.42c A2 0.50 60.00 1.72 ± 0.89bc 2.82 ± 0.85c A3 1.00 76.67 3.91 ± 1.65a 5.65 ± 1.18a A4 1.50 56.67 2.41 ± 1.28b 3.66 ± 0.94b A5 2.00 50.00 2.00 ± 0.65bc 3.53 ± 0.86b Mean 54.67 2.49 ± 1.49 3.87 ± 1.50 Note: Data are shown as mean ± SD. Data in each column followed by different letters were significantly different at p ≤ 0.05 using Duncan’s test. 27 DALAT UNIVERSITY JOURNAL OF SCIENCE [NATURAL SCIENCES AND TECHNOLOGY] The use of IBA also affects rooting similar to NAA (Table 2). All experimental treatments gave rooting parameters higher than the control. The best rooting treatment was IBA at 1.00% concentration, which gave a rooting percentage of 66.67%, 2.43 roots/cutting, and root lengths of 4.42 cm/cutting. The most effective concentration is followed by treatments with IBA at concentrations of 1.50%, 2.00%, and 0.50%. The control treatment has the lowest rooting efficiency. The most effective concentration of IAA of 1.50% (compared to 1.00% for NAA and IBA) produced a rooting percentage of 66.67%, 2.81 roots/cutting, and root lengths of 4.34 cm/cutting (Table 3). The next best treatments were 1.00% IAA and 2.00% IAA. The treatment of 0.50% IAA and the control treatment had similar results. Table 2. Influence of IBA on root formation of Actinidia latifolia cuttings Rooting Number of Length of Concentration Treatment percentage roots/cutting roots/cutting (%) (%) (cm) B1 Control 30.00 1.33 ± 0.50c 2.41 ± 0.42c B2 0.50 60.00 1.39 ± 0.61c 2.68 ± 0.40c B3 1.00 66.67 2.43 ± 1.25a 4.42 ± 0.85a B4 1.50 60.00 2.06 ± 0.54ab 4.29 ± 0.57a B5 2.00 36.67 1.82 ± 1.25ab 3.17 ± 0.48b Mean 54.67 1.88 ± 0.99 3.57 ± 1.02 Note: Data are shown as mean ± SD. Data in each column followed by different letters were significantly different at p ≤ 0.05 using Duncan’s test. Table 3. Influence of IAA on root formation of Actinidia latifolia cuttings Rooting Number of Length of Concentration Treatment percentage roots/cutting roots/cutting (%) (%) (cm) C1 Control 30.00 1.33 ± 0.50b 2.41 ± 0.42d C2 0.50 56.67 1.29 ± 0.47b 2.69 ± 0.61d C3 1.00 63.33 1.47 ± 0.51b 3.81 ± 0.58b C4 1.50 66.67 2.81 ± 1.21a 4.34 ± 0.57a C5 2.00 50.00 1.73 ± 0.88b 3.13 ± 0.62c Mean 53.33 1.81 ± 1.00 3.43 ± 0.91 Note: Data are shown as mean ± SD. Data in each column followed by different letters were significantly different at p ≤ 0.05 using Duncan’s test. Comparing the use of growth regulators in all three treatments, 1.0% NAA, 1.0% IBA, and 1.5% IAA, the rooting parameters were better, but more variable than with the control (Figure 2). PGRs should be used at appropriate doses because high concentrations inhibit rooting, similar to the above experiments (Tables 1-3). When NAA and IBA were used in concentrations of 1.5% and 2.0%, the rooting parameters were less than with the 28 Le Hong En, Hoang Thanh Truong, and Nguyen Thanh Nguyen 1.0% treatment. Similarly, concentrations over 1.5% should not be used with IAA. The above results are also consistent with previous research results (Nazir et al., 2018; Sağlam et al., 2014; Sun & Bassuk, 1991; Zhang et al. 2015). Figure 2. Influence of three plant growth regulators at various concentrations on root formation of semi-hardwood cuttings 3.2. Germination and growth of kiwifruit on sand substrates Results of kiwifruit sowing are shown in Table 4. Kiwifruit seeds sown on sand have a germination percentage of 81.00%. The seeds began to germinate after 19 days and germination ended after 25 days. The germination percentage can be increased by carefully selecting seeds, using germination stimulants, controlling the temperature, and choosing the right substrate. By so doing, the germination percentage reached 99.17% (Celik et al., 2006). Table 4. Germination and growth of kiwifruit on sand substrate Rooting percentage Height of seedlings Initial day of germination Final day of germination Substrate (%) (cm) (day) (day) Sand 81 1.0-1.2 19 25 3.3. Influence of substrates on kiwifruit growth When the seeds had germinated, hulled, and had two cotyledons, they were transplanted for the substrate mixing experiments. Plants are adapted to different soil types, with soil porosity and water-holding being important factors affecting plant growth. Table 5 shows growth results of kiwifruit seedlings for different soil-coconut coir combination ratios. The survival percentage is over 90% in all treatments. The most suitable soil and coconut coir mixture for kiwifruit seedlings is 25% soil mixed with 75% 29 DALAT UNIVERSITY JOURNAL OF SCIENCE [NATURAL SCIENCES AND TECHNOLOGY] coconut coir. The tallest seedlings and the highest number of leaves/seedling were 5.02 cm and 7.17 leaves, respectively. For the least effective treatment, 100% soil, the plant height was only 2.10 cm and seedlings had only 3.88 leaves on average. The variations in the number of leaves and plant height in the experimental treatments are expressed in the standard deviation values (Figure 3). The standard deviation of the number of leaves in the treatments ranged from 0.40 to 0.52 (except for treatment D2, which was 0.24). The standard deviation of plant height ranged from 0.61 to 0.80. These initial results show that the nursery-stage kiwifruit seedlings are suited to soils with high porosity and moisture content rather than compact, low-moisture soils. The growth results of the substrate treatments are shown clearly in Figure 4. Seedlings in the nursery grew and adapted well to the weather conditions in Da Lat (Lam Dong province) (Figure 5). Table 5. Influence of substrates on kiwifruit growth Treatment Living percentage Height of seedling Number of Symbol Soil Coconut coir (%) (cm) leaves/seedling (%) (%) D1 100 0 96.00 2.10 ± 0.41e 3.88 ± 0.80d D2 75 25 96.00 2.66 ± 0.24d 5.17 ± 0.70c D3 50 50 100.00 3.28 ± 0.44c 5.96 ± 0.61b D4 25 75 96.00 5.02 ± 0.40a 7.17 ± 0.64a D5 0 100 92.00 4.28 ± 0.52b 6.30 ± 0.70b Mean 96.00 3.46 ± 1.14 5.69 ± 1.31 Note: Data are shown as mean ± SD. Data in each column followed by different letters were significantly different at p ≤ 0.05 using Duncan’s test. Figure 3. Height and number of kiwifruit seedling leaves for various experimental treatments 30 Le Hong En, Hoang Thanh Truong, and Nguyen Thanh Nguyen (a) (b) (c) (d) (e) (f) Figure 4. Growth of kiwifruit seedlings for various experimental substrates Note: (a) Mixed soil and coconut coir substrates, from left to right: 0%, 25%, 50%, 75%, and 100% coconut coir; (b) Control (0% coconut coir); (c) 25% coconut coir; (d) 50% coconut coir; (e) 75% coconut coir; (f) 100% coconut coir. Figure 5. Growth of kiwifruit seedlings after three months 4. CONCLUSIONS The results of this study show that PGRs can promote the root formation of Actinidia latifolia in propagation by cuttings. Using a concentration of 1.0% for NAA and IBA and a concentration of 1.5% for IAA showed the strongest effect on root growth. The germination percentage of kiwifruit seeds reached 81.0% after 25 days. The best substrate for the growth of kiwifruit seedlings is 25.0% soil with 75.0% coconut coir. 31 DALAT UNIVERSITY JOURNAL OF SCIENCE [NATURAL SCIENCES AND TECHNOLOGY] ACKNOWLEDGMENTS The authors would like to thank the Forest Science Institute of Central Highlands and South of Central Vietnam. They created the most favorable conditions for us to complete this study. REFERENCES Celik, H., Zenginbal, H., & Özcan, M. (2006). Enghancing germination of kiwifruit seeds with temperature, medium and gibberelic acid. Horticultural Science, 33(1), 39-45. Du, G., Li, M., Ma, F., & Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chemistry, 113(2), 557-562. Duncan, D. B. (1955). Multipe range and multiple F tests. Biometrics, 11, 1-42. Duong, N. T., Tran, H. T., Trinh, H. T., Hoang, C. V., & Nguyen, H. P. (2012). Study the formation of callus and single cell of Kiwi (Actinidia deliciosa). Journal of Biology, 34(4), 505-514. Huang, H. (2016). Kiwifruit: The genus Actinidia (1st Edition). Academic Press. Kulevanona, S. (2011). Important medicinal and aromatic plants in Southeast Europe in relation with their medicinal and other industrial utilization: Republic of Macedonia. Institute of Organic Chemistry with Centre of Phytochemistry, Bulgaria. Nazir, N., Kamili, A. N., Shah, D., & Zargar, M. Y. (2018). Adventitious rooting in shoot cuttings of Taxus wallichiana zucc., an endangered medicinally important conifer of Kashmir Himalaya. Forest Research, 7(2), 1-6. Phạm, H. H. (2000). Cây cỏ Việt Nam (An illustrated flora of Vietnam) (Quyển 1). NXB Trẻ. Richardson, D. P., Ansell, J., & Drummond, L. N. (2018). The nutritional and health attributes of kiwifruit: A review. European Journal of Nutrition, 57(8), 2659-2676. Sağlam, A. C., Yaver, S., Başer, I., & Cinkiliç, L. (2014). The effects of different hormones and their doses on rooting of stem cuttings in Anatolian Sage (Salvia fruticosa mill.). APCBEE Procedia, 8, 348-353. Sun, W. Q., & Bassuk, N. (1991). Does IBA inhibit shoot growth in rooted cuttings? Combined Proceedings International Plant Propagators’ Society, 41, 456-461. Üçler, A. Ö., Parlak, S., & Yücesan, Z. (2004). Effects of IBA and cutting dates on the rooting ability of semi-hardwood kiwifruit Actinidia deliciosa A. chev. cuttings. Turkish Journal of Agriculture and Forestry, 28(3), 195-201. 32 Le Hong En, Hoang Thanh Truong, and Nguyen Thanh Nguyen Võ, C. V. (2012). Từ điển cây thuốc Việt Nam (Dictionary of medicinal plants in Vietnam) (Tập 1). NXB Y học. Zhang, J. Y., Guo, Z. R., Zhang, R., Li, Y. R., Cao, L., Liang, Y. W., & Huang, L. B. (2015). Auxin type, auxin concentration, and air and substrate temperature difference play key roles in the rooting of juvenile hardwood pecan cuttings. HortTechnology, 25(2), 209-213. 33
File đính kèm:
 study_on_the_propagation_of_actinidia_latifolia_and_actinidi.pdf
study_on_the_propagation_of_actinidia_latifolia_and_actinidi.pdf

