Reduction of graphene oxide in ethanol solution by gamma irradiation for preparing reduced graphene oxide material with water desalination
Reduction of graphene oxide (GO) for preparing the reduced graphene oxide (rGO) by γ–
ray irradiation was investigated. GO was dispersed in the ethanol solution with the GO concentration
of 1 mg/ml, then irradiated with γ–ray in presence of oxygen at dose range of 0 – 100 kGy for
preparation of rGO product. The characteristic properties of GO and rGO were determined by UV-Vis
spectroscopy, X–ray diffraction (XRD), Transmission Electron Microscopy (TEM), contact angle
measurement and test of water desalination. The result showed that water desalination efficiency of
rGO was about 46 – 48%

Trang 1

Trang 2
Trang 3
Trang 4
Trang 5

Trang 6

Trang 7
Bạn đang xem tài liệu "Reduction of graphene oxide in ethanol solution by gamma irradiation for preparing reduced graphene oxide material with water desalination", để tải tài liệu gốc về máy hãy click vào nút Download ở trên
Tóm tắt nội dung tài liệu: Reduction of graphene oxide in ethanol solution by gamma irradiation for preparing reduced graphene oxide material with water desalination

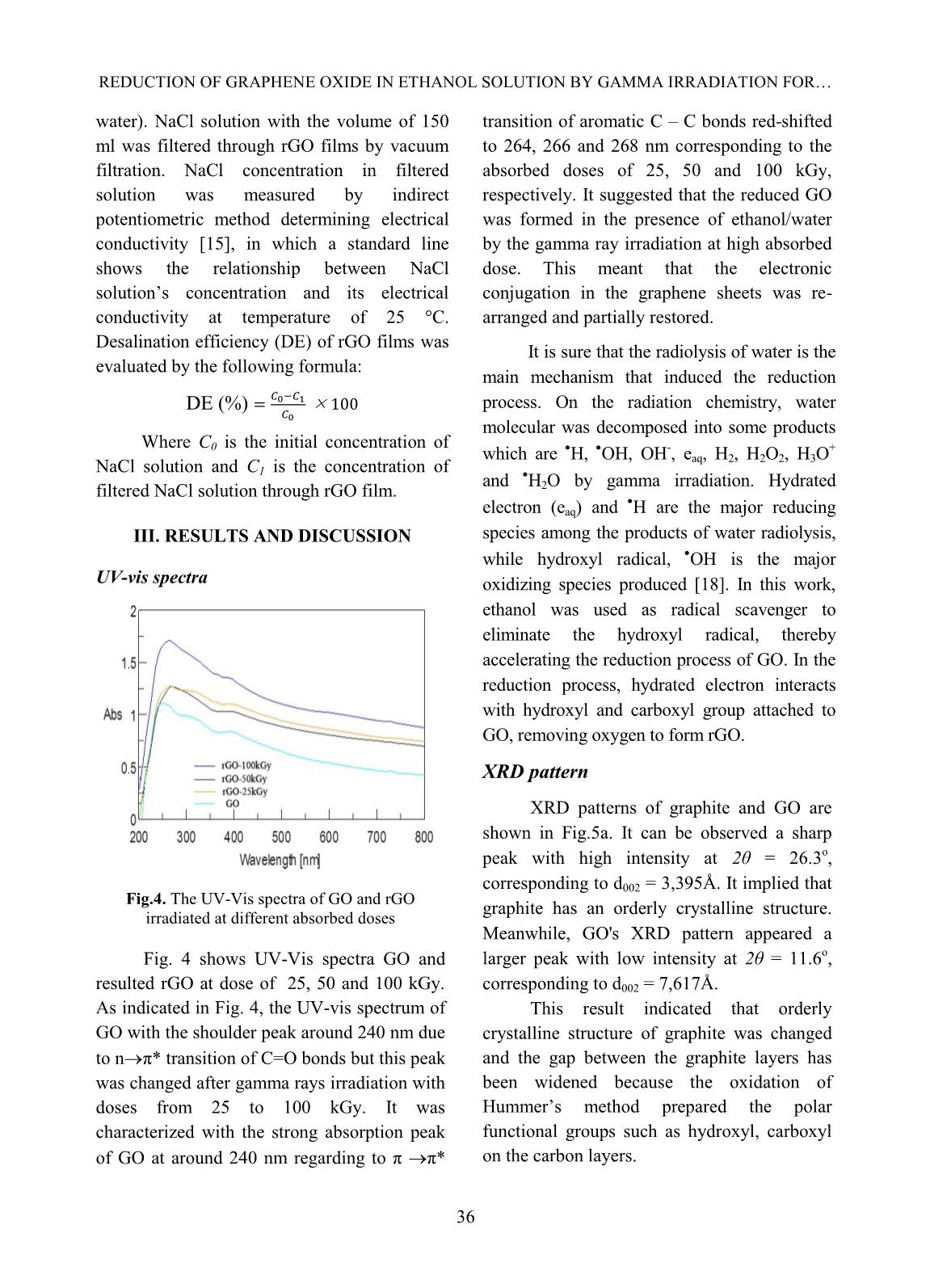
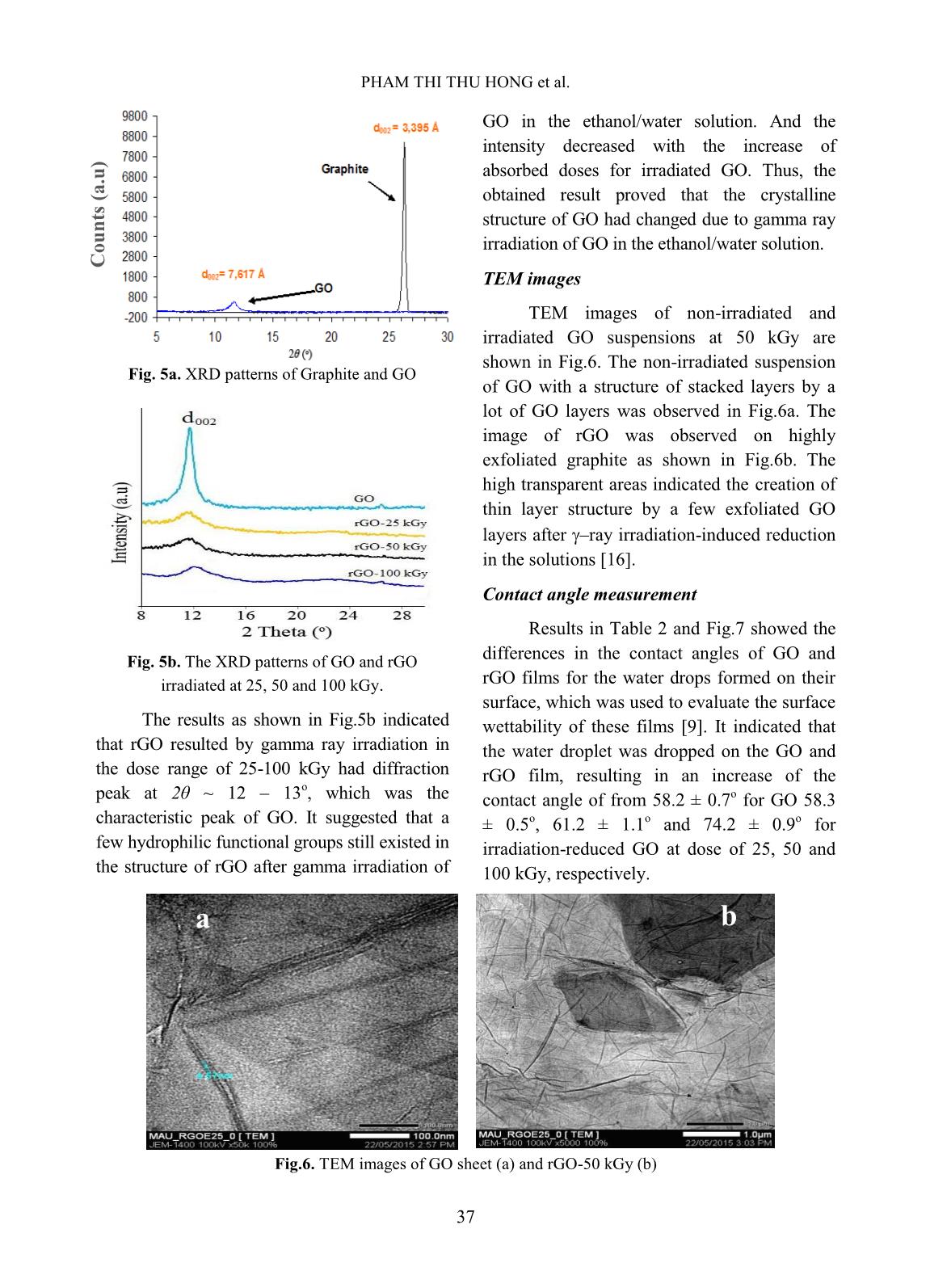
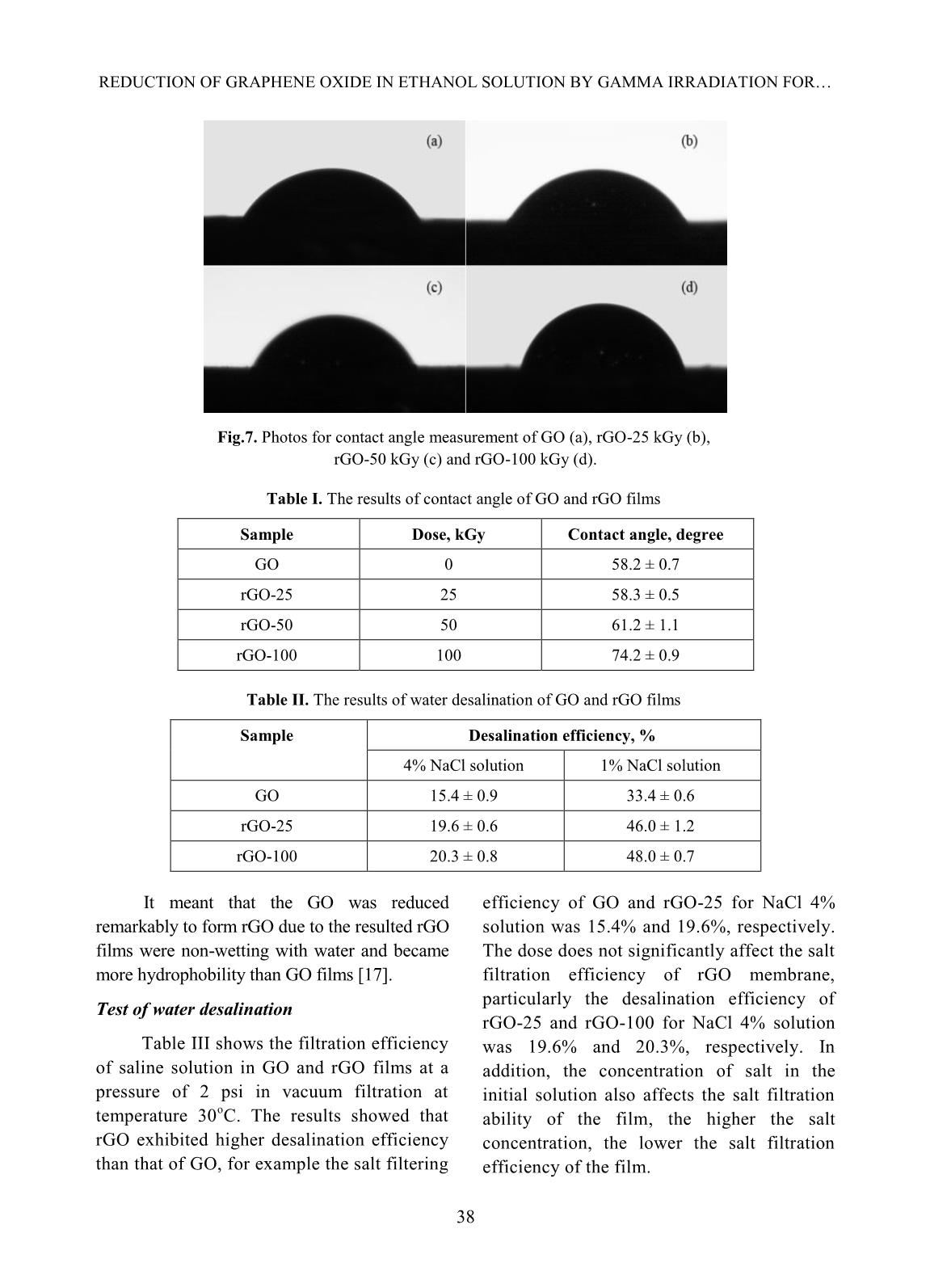
esalination efficiency of rGO was about 46 – 48%. Keywords: graphene oxide, gamma, irradiation, desalination. I. INTRODUCTION Graphene can be defined as a mono- layer of carbon atoms connected by the sp 2 bonds forming the hexagonal lattice in structure [1] (Fig.1). It was discovered by two scientists of Novoselov and Gein (2004) with its attractive properties including good thermal conductivity, large theoretical specific surface area, high electron mobility, good optical transmittance; in addition, it is so thin and harder than diamonds [1,2]. Until now, graphene has been studied for application in many high-technique fields such as bio-sensor, solar cells, energy storage and so on [1,2]. Fig.1. Structure of graphene material In recent years, graphene material was prepared by reduction process of graphene oxide (GO) through many different methods: chemical, solvothermal, electrochemical method, ultrasonic irradiation, gamma rays or electron beam irradiation and so on [3-11]. And GO is usually synthesized via an oxidizing process of graphite using oxidants based on Hummers’method and modified Hummers’approach [5]. There are lots of reduction routes of GO sheets to produce rGO with a good improvement of its properties as graphene’s ones. One of useful properties of graphene and GO is its ability of water desalination [12-14]. In this work, we focus on the reduction of GO to prepare reduced GO (rGO) by gamma rays irradiation in ethanol/water medium to improve the ability of desalination aiming at the development and production of membrane filter applied in treatment of salt water or brackish water. PHAM THI THU HONG et al. 35 II. EXPERIMENTAL Sample irradiation to prepare rGO Graphite oxide was synthesized from graphite powder (SEC Carbon, Japan) by the modified Hummer’s method [5, 11]. GO suspension was prepared by adding an amount of 0.01 g of GO in 10 ml of ethanol/water solution (25%, v/v) and dispersed under ultrasonic for 1 hour in an ultrasonic bath (Elma Elmasonic S40H, Germany). The prepared suspension was sealed and irradiated at a room temperature by gamma Co-60 rays in the range of absorbed doses from 0 to 100 kGy with the dose rate of 1.0 kGy/h. Irradiated GO (or rGO) was filtrated and washed by distilled water several times, dried at 70 o C and ground into a fine powder for next experiments. Fig. 2. Suspensions of GO and rGO irradiated at doses of 25, 50 and 100 kGy Preparation of rGO films The rGO films were prepared by dispersing 0.04 g of rGO in 200 ml of ethanol solvent under ultrasonic for 2 hours in the ultrasonic bath. Then, the suspension was centrifuged at 2000 rpm by a centrifuge (EBA 12, Hettich, Germany) and decanted the supernatant. rGO films were obtained by vacuum filtration of the prepared rGO with an alpha-cellulose membrane, washing with distilled water several times, dried at 80 o C for 48 hours and kept in a desiccator before use. Fig. 3. rGO film (a) and rGO films on cellulose acetate membrane (b) Characterization - UV-vis absorption spectra were measured with a JASCO V630 spectrophotometer, Japan in the wavelength region of 200 – 800 nm. Gamma ray pre- irradiated and post-irradiated GO samples in ethanol/water solution were prepared at a concentration of 0.025 mg/ml. - X-ray diffraction patterns of the samples were taken on a XRD D8 Advance diffractometer, Bruker, Germany with monochromatized Cu-K radiation (l= 1.5406Å) at a scanning rate of 0.04 o /s in a wide angle range (2q = 5 - 30 o ) at ambient temperature. - The TEM measurements of all samples were performed on a JEM 1010 instrument, JEOL, Japan with the high resolution scanning electron micro-scope plus a transmission mode. - Contact angle measurement: A rGO film was prepared with diameter of 33 millimeters and weight of 20 mg. Surface wettability of all sample films was measured on an OCA 20L instrument (Germany) using distilled water as a wettable solvent in condition of room temperature. - Test of water desalination: NaCl solution was prepared at the concentration of 4% (like the salt concentration in seawater) and 1% (like the salt concentration in brackish REDUCTION OF GRAPHENE OXIDE IN ETHANOL SOLUTION BY GAMMA IRRADIATION FOR 36 water). NaCl solution with the volume of 150 ml was filtered through rGO films by vacuum filtration. NaCl concentration in filtered solution was measured by indirect potentiometric method determining electrical conductivity [15], in which a standard line shows the relationship between NaCl solution’s concentration and its electrical conductivity at temperature of 25 °C. Desalination efficiency (DE) of rGO films was evaluated by the following formula: DE (%) Where C0 is the initial concentration of NaCl solution and C1 is the concentration of filtered NaCl solution through rGO film. III. RESULTS AND DISCUSSION UV-vis spectra Fig.4. The UV-Vis spectra of GO and rGO irradiated at different absorbed doses Fig. 4 shows UV-Vis spectra GO and resulted rGO at dose of 25, 50 and 100 kGy. As indicated in Fig. 4, the UV-vis spectrum of GO with the shoulder peak around 240 nm due to n π* transition of C=O bonds but this peak was changed after gamma rays irradiation with doses from 25 to 100 kGy. It was characterized with the strong absorption peak of GO at around 240 nm regarding to π π* transition of aromatic C – C bonds red-shifted to 264, 266 and 268 nm corresponding to the absorbed doses of 25, 50 and 100 kGy, respectively. It suggested that the reduced GO was formed in the presence of ethanol/water by the gamma ray irradiation at high absorbed dose. This meant that the electronic conjugation in the graphene sheets was re- arranged and partially restored. It is sure that the radiolysis of water is the main mechanism that induced the reduction process. On the radiation chemistry, water molecular was decomposed into some products which are H, OH, OH - , eaq, H2, H2O2, H3O + and H2O by gamma irradiation. Hydrated electron (eaq) and H are the major reducing species among the products of water radiolysis, while hydroxyl radical, OH is the major oxidizing species produced [18]. In this work, ethanol was used as radical scavenger to eliminate the hydroxyl radical, thereby accelerating the reduction process of GO. In the reduction process, hydrated electron interacts with hydroxyl and carboxyl group attached to GO, removing oxygen to form rGO. XRD pattern XRD patterns of graphite and GO are shown in Fig.5a. It can be observed a sharp peak with high intensity at 2θ = 26.3o, corresponding to d002 = 3,395Å. It implied that graphite has an orderly crystalline structure. Meanwhile, GO's XRD pattern appeared a larger peak with low intensity at 2θ = 11.6o, corresponding to d002 = 7,617Å. This result indicated that orderly crystalline structure of graphite was changed and the gap between the graphite layers has been widened because the oxidation of Hummer’s method prepared the polar functional groups such as hydroxyl, carboxyl on the carbon layers. PHAM THI THU HONG et al. 37 Fig. 5a. XRD patterns of Graphite and GO Fig. 5b. The XRD patterns of GO and rGO irradiated at 25, 50 and 100 kGy. The results as shown in Fig.5b indicated that rGO resulted by gamma ray irradiation in the dose range of 25-100 kGy had diffraction peak at 2θ ~ 12 – 13o, which was the characteristic peak of GO. It suggested that a few hydrophilic functional groups still existed in the structure of rGO after gamma irradiation of GO in the ethanol/water solution. And the intensity decreased with the increase of absorbed doses for irradiated GO. Thus, the obtained result proved that the crystalline structure of GO had changed due to gamma ray irradiation of GO in the ethanol/water solution. TEM images TEM images of non-irradiated and irradiated GO suspensions at 50 kGy are shown in Fig.6. The non-irradiated suspension of GO with a structure of stacked layers by a lot of GO layers was observed in Fig.6a. The image of rGO was observed on highly exfoliated graphite as shown in Fig.6b. The high transparent areas indicated the creation of thin layer structure by a few exfoliated GO layers after –ray irradiation-induced reduction in the solutions [16]. Contact angle measurement Results in Table 2 and Fig.7 showed the differences in the contact angles of GO and rGO films for the water drops formed on their surface, which was used to evaluate the surface wettability of these films [9]. It indicated that the water droplet was dropped on the GO and rGO film, resulting in an increase of the contact angle of from 58.2 ± 0.7 o for GO 58.3 ± 0.5 o , 61.2 ± 1.1 o and 74.2 ± 0.9 o for irradiation-reduced GO at dose of 25, 50 and 100 kGy, respectively. Fig.6. TEM images of GO sheet (a) and rGO-50 kGy (b) C o u n ts ( a .u ) a b REDUCTION OF GRAPHENE OXIDE IN ETHANOL SOLUTION BY GAMMA IRRADIATION FOR 38 Fig.7. Photos for contact angle measurement of GO (a), rGO-25 kGy (b), rGO-50 kGy (c) and rGO-100 kGy (d). Table I. The results of contact angle of GO and rGO films Sample Dose, kGy Contact angle, degree GO 0 58.2 ± 0.7 rGO-25 25 58.3 ± 0.5 rGO-50 50 61.2 ± 1.1 rGO-100 100 74.2 ± 0.9 Table II. The results of water desalination of GO and rGO films Sample Desalination efficiency, % 4% NaCl solution 1% NaCl solution GO 15.4 ± 0.9 33.4 ± 0.6 rGO-25 19.6 ± 0.6 46.0 ± 1.2 rGO-100 20.3 ± 0.8 48.0 ± 0.7 It meant that the GO was reduced remarkably to form rGO due to the resulted rGO films were non-wetting with water and became more hydrophobility than GO films [17]. Test of water desalination Table III shows the filtration efficiency of saline solution in GO and rGO films at a pressure of 2 psi in vacuum filtration at temperature 30 o C. The results showed that rGO exhibited higher desalination efficiency than that of GO, for example the salt filtering efficiency of GO and rGO-25 for NaCl 4% solution was 15.4% and 19.6%, respectively. The dose does not significantly affect the salt filtration efficiency of rGO membrane, particularly the desalination efficiency of rGO-25 and rGO-100 for NaCl 4% solution was 19.6% and 20.3%, respectively. In addition, the concentration of salt in the initial solution also affects the salt filtration ability of the film, the higher the salt concentration, the lower the salt filtration efficiency of the film. PHAM THI THU HONG et al. 39 IV. CONLUSIONS Graphene oxide can be simply reduced and exfoliated by gamma Co-60 ray irradiation in alcohol/water solution. The transparent solution of rGO was obtained after gamma irradiation-induced reduction, which was confirmed by analysis of rGO through UV-Vis spectra, XRD pattern and TEM images. Hydrophobic properties of rGO increased steadily with the increase of absorbed dose. Desalination ability of rGO film was better than that of GO one. Further research should be carried out to analyze the effect of filtration pressure and desalination capacity of rGO film. ACKNOWLEGEMENTS The authors would like to thanks VINAGAMMA Center for helping in gamma irradiation. REFERENCES [1]. Royal Swedish Academy of Sciences, Scientific Background on the Nobel Prize in Physics 2010 GRAPHENE, 2010. [2]. Y. Zhu, “Graphene and graphene oxide: Synthesis, properties, and application”, Advanced Materials, 22, 1-19, 2010. [3]. D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L.B. Alemany, W.L., and J.M. Tour, “Improved synthesis of graphene oxide”, ACS Nano, 4, 4806-4814, 2010. [4]. W.S. Haummers, Jr., and R.E. Offeman, “Preparation of graphitic oxide”, Journal of the American Chemical Society, 80 (6), 1958. [5]. Tam T. Mai, Chi Nhan Ha Thuc and Huy Ha Thuc, “Preparation of Graphene Nano-Layer by Chemical Graphitization of Graphite Oxide from Exfoliation and Preliminary Reduction”, Fullerenes, Nanotubes and Carbon Nanostructures, 23, 742-749, 2015. [6]. C. Xu, R. Yuan, and X. Wang, “Selective reduction of graphene oxide”, New Carbon Materials, 29, 61-66, 2014. [7]. X. Li, T. Tang, M. Li, and X. He, “Photochemical doping of graphene oxide thin films with nitrogen for electrical conductivity improvement”, Journal of Material Science: Materials in Electronics, 26, 1770-1775, 2015. [8]. V. Singh, D. Joung, L. Zhai, S. Das, S.I. Khondaker, and S. Seal, “Graphene based materials: Past, present and future”, Progress in Materials Science, 56, 1178-1271, 2011. [9]. Z. Wang, P. Li, Y. Chen, J. He, B. Zheng, J. Liu, and F. Qi, “The green synthesis of reduced graphene oxide by the ethanol-thermal reaction and its electrical properties”, Materials Letters, 116, 416-419, 2014. [10]. M. Park, H.K. Shin, B.S. Kim, B. Pant, N.A.M. Bakakat, and H.Y. Kim, “Facile preparation of graphene induced from electron- beam irradiated graphite”, Materials Letters, 105, 236-238, 2013. [11]. P. T. T. Hong, “Study on synthesizing of graphene material from graphene oxide by Co-60 gamma irradiation method”, Master thesis, University of Engineering and Technology, Vietnam National University, Hanoi, 2015. [12]. H. M. Hegab and L. Zou, “Graphene oxide- assisted membranes: Fabrication and potential applications in desalination and water purification”, Journal of Membrane Science, 484, 95-106, 2015. [13]. E. Y. M. Ang, T. Y. Ng, J. Yeo, Z. Liu and K. R. Geethalakshmi, “Free-standing graphene slit membrane for enhanced desalination”, Carbon, 110, 350-355, 2016. [14]. S. M. Ghaseminezhad, M. Barikani, M. Salehirad, “Development of graphene oxide- cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination”, Composites Part B, 161, 320-327, 2019. [15]. A. Aghigh, V. Alizadeh, H. Y. Wong, Md. S. Islam, N. Amin and M. Zaman, “Recent advances in utilization of graphene for REDUCTION OF GRAPHENE OXIDE IN ETHANOL SOLUTION BY GAMMA IRRADIATION FOR 40 filtration and desalination of water: A review”, Desalination, 365, 389-397, 2015. [16]. L. Stobinski and et al., “Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods”. Journal of Electron Spectroscopy and Related Phenomena, 195, 145-154, 2014. [17]. W. A. Zisman, “Relation of the equilibrium contact angle to liquid and solid constitution”, Advances in Chemistry, 43, 1- 51, 1964. [18]. K.A. Khan, “The Radiation Chemistry of water”. Journal of Physic and Chemistry, 105- 110, 1981.
File đính kèm:
 reduction_of_graphene_oxide_in_ethanol_solution_by_gamma_irr.pdf
reduction_of_graphene_oxide_in_ethanol_solution_by_gamma_irr.pdf

