Study on the preparation of selenium nanoparticles by gamma Co-60 method and investigate the stability
Among nanoparticle materials, selenium nanoparticles (SeNPs) have attracted wide spread
attention due to their excellent bioavailability, high bioactivity and low toxicity compared to other
ionic selenium compounds. SeNPs with size ~ 41.75 nm were synthesized by γ-irradiation method
using oligochitosan (OC) as stabilizer. The prepared SeNPs/OC were characterized by UV-Vis
spectroscopy and transmission electron microscope (TEM) images. The SeNPs/OC powder was also
prepared by spray drying technique and the purity was verified by energy dispersive X-ray (EDX)
analysis. The results of EDX showed that SeNPs/OC solution was of high purity. The stability of
SeNPs/OC solution was investigated. The results indicated that SeNPs/OC solution had good stability
after 60 days of storage at 4ºC. At ambient temperature, the SeNPs/OC solution was unstable and
agglomerated after about 15 days. The SeNPs/OC synthesized by γ-irradiation with the advantages of
environmental friendly and mass production process may be potentially promising for applications in
medicines, functional food and in other fields as well.

Trang 1

Trang 2
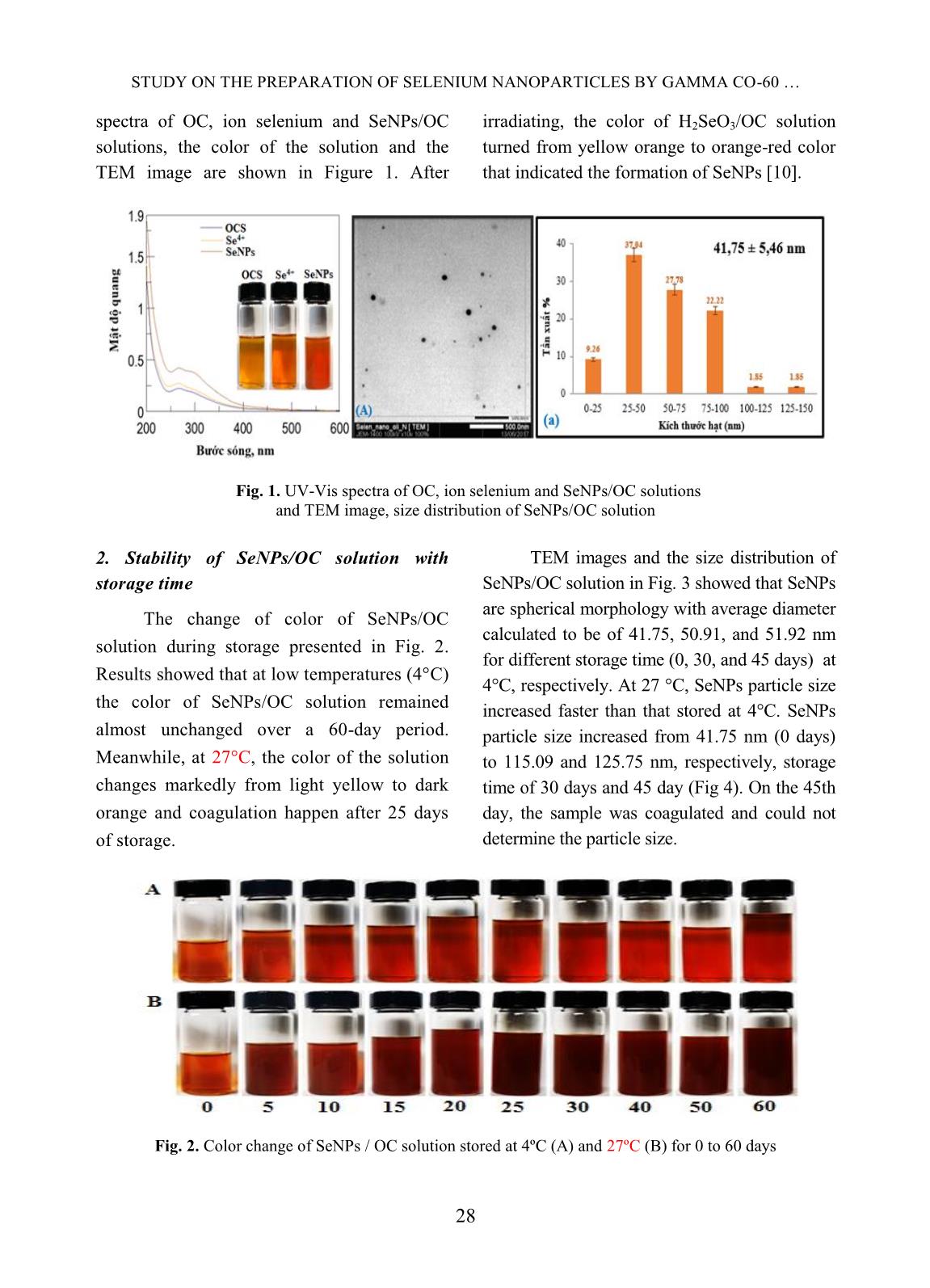
Trang 3
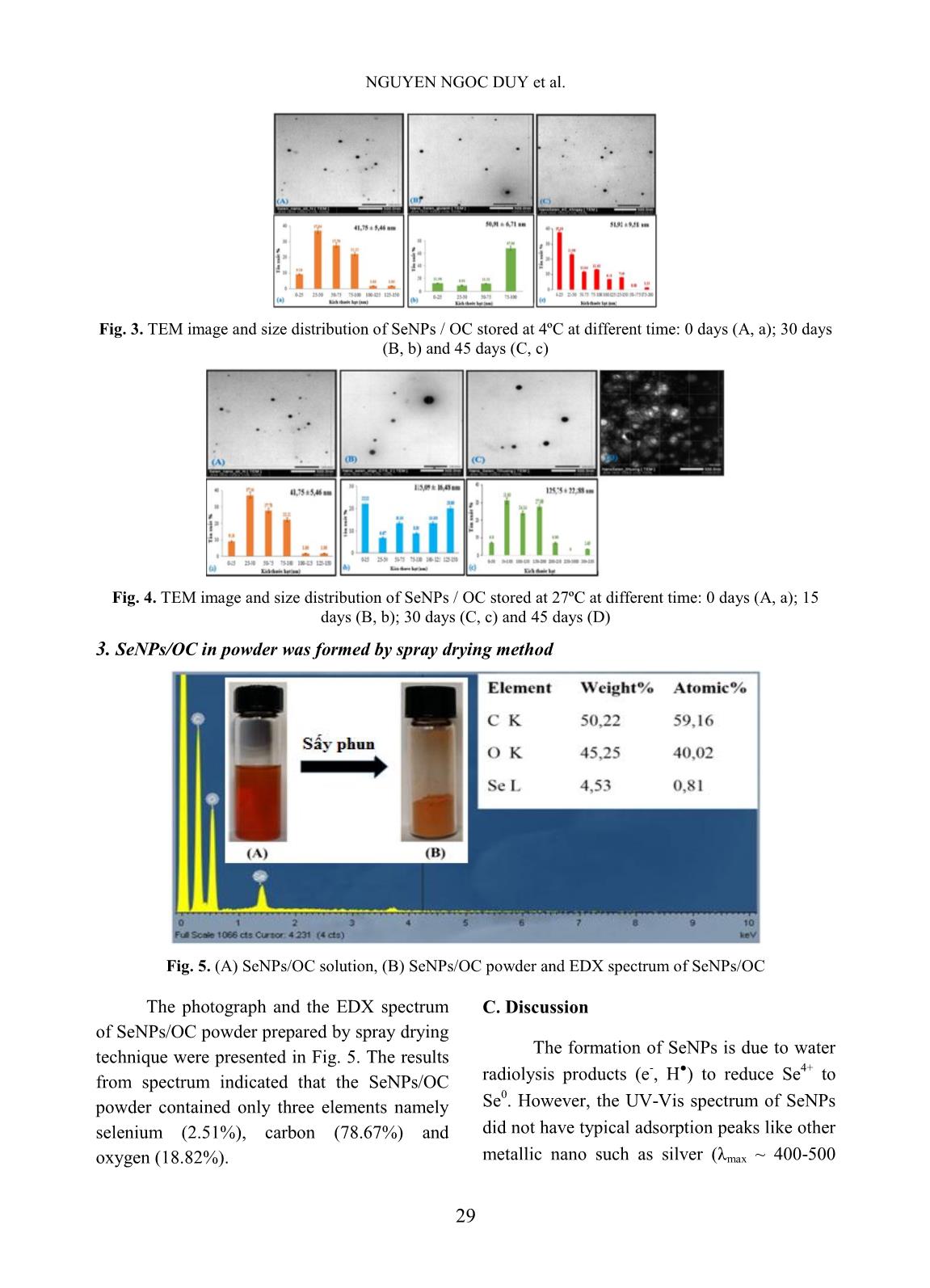
Trang 4

Trang 5

Trang 6
Tóm tắt nội dung tài liệu: Study on the preparation of selenium nanoparticles by gamma Co-60 method and investigate the stability

Nuclear Science and Technology, Vol.10, No. 2 (2020), pp. 26-31 ©2020 Vietnam Atomic Energy Society and Vietnam Atomic Energy Institute Study on the preparation of selenium nanoparticles by gamma Co-60 method and investigate the stability Ngoc Duy Nguyen 1 , Van Phu Dang 1 , Anh Quoc Le 1 , T. Kim Lan Nguyen 1 , Quoc Hien Nguyen 1 , T. Thu Ngan Tran 2 1 Research and Development Center for Radiation Technology, 202A, Street 11, Thu Duc District, Ho Chi Minh City, Vietnam 2 University of Science, VNU-HCM, 227 Nguyen Van Cu, District 5, Ho Chi Minh City, Vietnam Email: ngocduy158@gmail.com (Received 26 November 2019, accepted 31 March 2020) Abstract: Among nanoparticle materials, selenium nanoparticles (SeNPs) have attracted wide spread attention due to their excellent bioavailability, high bioactivity and low toxicity compared to other ionic selenium compounds. SeNPs with size ~ 41.75 nm were synthesized by γ-irradiation method using oligochitosan (OC) as stabilizer. The prepared SeNPs/OC were characterized by UV-Vis spectroscopy and transmission electron microscope (TEM) images. The SeNPs/OC powder was also prepared by spray drying technique and the purity was verified by energy dispersive X-ray (EDX) analysis. The results of EDX showed that SeNPs/OC solution was of high purity. The stability of SeNPs/OC solution was investigated. The results indicated that SeNPs/OC solution had good stability after 60 days of storage at 4ºC. At ambient temperature, the SeNPs/OC solution was unstable and agglomerated after about 15 days. The SeNPs/OC synthesized by γ-irradiation with the advantages of environmental friendly and mass production process may be potentially promising for applications in medicines, functional food and in other fields as well. Keywords: Selenium nanoparticles, γ-irradiation, oligochitosan. I. INTRODUCTION Cancer is now the leading cause of death worldwide. According to estimation by the World Cancer Research Agency (IARC), there were 14.1 million new cancer cases and 8.2 million deaths in 2012. Radiotherapy and chemotherapy are still considered to be the most optimal measures, but they also cause many unwanted side effects such as the severely reduced number of blood cells, which can cause anemia and infection with opportunistic microorganisms caused by the weakened immune system [1]. Selenium is an important trace element, which has broad effects on biological systems, including antioxidant effect, cancer prevention and antiviral activitiy [8]. The necessary selenium content in the diet of adults is 50 - 200 μg/day [2]. Compared to selenium in ionic form, SeNPs have higher bioavailability and lower toxicity [3]. Result of the previous studies showed that SeNPs have a much lower acute toxicity in mice with LD50 ~ 91.2 mg Se/kg body weight compared to methylselenocysteine with LD50 ~ 14.6 mg Se/kg body weight [4]. Recently, Zhai et al. [5] also reported that the LD50 for SeNPs for Kunming mice was 258.2 mg/kg while the LD50 for H2SeO3 was 22 mg/kg. In addition, studies have shown that SeNPs were effective in treating cancer. Sonkusre et al. [6] have demonstrated that SeNPs were highly effective and specific against prostate cancer. Ali et al. found that mice supplemented SeNPs (50 - 80 nm) at a dose of 0.2 mg/kg body weight were able to NGUYEN NGOC DUY et al. 27 fight lung cancer [7]. Faghfuri et al. [8] reported that breast tumor in mice supplemented with 200 μg SeNPs/day for 60 days was smaller than the control group that did not use SeNPs. Recently, Zhai et al. [6] also reported that the LD50 of SeNPs for Kunming mice was 258.2 mg/kg while the LD50 for H2SeO3 was 22 mg/kg. Several methods have been applied to synthesize SeNPs from Se ions such as chemical reduction methods using ascorbic acid, glutathione, hydrazine hydrate, etc. as reducing agents [3, 4], biological methods. using bacterial biomass as a reducing agent [7, 8], the gamma Co-60 irradiation method used sodium dodecyl sulfate as a stabilizer and ethanol as a free radical capture agent [9, 10]. In particular, irradiation method is considered as an effective method to synthesize SeNPs with advantages such as: (1) the reaction is performed at room temperature, (2) the efficiency of creating high SeNPs, (3) SeNPs are of high purity due to the absence of reductant residues, (4) easily adjust SeNPs particle size by changing the dose and dose rate, (5) capable of producing in large quantities [9, 10]. In this study, SeNPs were synthesized by gamma Co-60 irradiation method using OC as a stabilizer. The stability of SeNPs/OC solution during storage was investigated. II. CONTENT A. Subjects and methods 1. Chemicals Selenium dioxide (SeO2) was of pure product of Merck, Germany. OC solution is a product of the Research and Development Center for Radiation Technology (VINAGAMMA) with the concentration of 3%, deacetyl ~ 85% and Mw~ 5000 g/mol. Other chemicals were of pure grade. Distilled water was used throughout the experiments. 2. Preparation of SeNPs/OC by γ-irradiation A required amount of SeO2 was dissolved in 1% (w/v) OC solution to prepare selenous acid (H2SeO3) solution (eq. (1)) with concentration of 2.5 mM. SeO2(s) + H2O(l) → H2SeO3(aq) (1) Irradiation of SeO3 2- /OC solutions to synthesize SeNPs was carried out on a Gamma Co-60 SVST at VINAGAMMA at dose of 20 kGy, with the dose rate of 1.3 kGy/h measured by a dichromate dosimetry system [10]. 3. Characterization and stability of SeNPs/OC The absorption spectra of OC and the resulted SeNPs/OC solutions were taken on an UV-Vis spectrophotometer model UV-2401PC (Shimadzu, Japan). The size and size distribution of the SeNPs were characterized by TEM images on transmission electron microscope (TEM), model JEM1010 (JEOL, Japan) and statistically calculated from about 300 particles [10]. The SeNPs/OC powder was prepared by spray drying of 2.5 mM SeNPs/1% OC solution with spray dryer model ADL311 (Yamato, Japan). The content of selenium in SeNPs/OC powder was assessed by energy dispersive X-ray (EDX) spectroscopy on a JEOL 6610 LA. The stability of SeNPs/OC solution determined by changes in particle size with storage time. B. Results 1. Characteristics of SeNPs /OC solution Nano selenium was prepared by the gamma-Co-60 irradiation method with a dose of 20 kGy, using 2% OC as a stabilizer according to Hien et al. [10]. The UV-Vis STUDY ON THE PREPARATION OF SELENIUM NANOPARTICLES BY GAMMA CO-60 28 spectra of OC, ion selenium and SeNPs/OC solutions, the color of the solution and the TEM image are shown in Figure 1. After irradiating, the color of H2SeO3/OC solution turned from yellow orange to orange-red color that indicated the formation of SeNPs [10]. Fig. 1. UV-Vis spectra of OC, ion selenium and SeNPs/OC solutions and TEM image, size distribution of SeNPs/OC solution 2. Stability of SeNPs/OC solution with storage time The change of color of SeNPs/OC solution during storage presented in Fig. 2. Results showed that at low temperatures (4°C) the color of SeNPs/OC solution remained almost unchanged over a 60-day period. Meanwhile, at 27°C, the color of the solution changes markedly from light yellow to dark orange and coagulation happen after 25 days of storage. TEM images and the size distribution of SeNPs/OC solution in Fig. 3 showed that SeNPs are spherical morphology with average diameter calculated to be of 41.75, 50.91, and 51.92 nm for different storage time (0, 30, and 45 days) at 4°C, respectively. At 27 °C, SeNPs particle size increased faster than that stored at 4°C. SeNPs particle size increased from 41.75 nm (0 days) to 115.09 and 125.75 nm, respectively, storage time of 30 days and 45 day (Fig 4). On the 45th day, the sample was coagulated and could not determine the particle size. Fig. 2. Color change of SeNPs / OC solution stored at 4ºC (A) and 27ºC (B) for 0 to 60 days NGUYEN NGOC DUY et al. 29 Fig. 3. TEM image and size distribution of SeNPs / OC stored at 4ºC at different time: 0 days (A, a); 30 days (B, b) and 45 days (C, c) Fig. 4. TEM image and size distribution of SeNPs / OC stored at 27ºC at different time: 0 days (A, a); 15 days (B, b); 30 days (C, c) and 45 days (D) 3. SeNPs/OC in powder was formed by spray drying method Fig. 5. (A) SeNPs/OC solution, (B) SeNPs/OC powder and EDX spectrum of SeNPs/OC The photograph and the EDX spectrum of SeNPs/OC powder prepared by spray drying technique were presented in Fig. 5. The results from spectrum indicated that the SeNPs/OC powder contained only three elements namely selenium (2.51%), carbon (78.67%) and oxygen (18.82%). C. Discussion The formation of SeNPs is due to water radiolysis products (e - , H ● ) to reduce Se 4+ to Se 0 . However, the UV-Vis spectrum of SeNPs did not have typical adsorption peaks like other metallic nano such as silver (λmax ~ 400-500 STUDY ON THE PREPARATION OF SELENIUM NANOPARTICLES BY GAMMA CO-60 30 nm) nad gold (λmax ~ 520-570 nm). According to Lin and Wang [11], the SeNPs with diameter less than 100 nm do not have characteristic absorption peak (λmax) in the UV-Vis region (200-800 nm). TEM images and the size distribution of SeNPs in Fig. 1 (A, a) showed that SeNPs are spherical morphology with average diameter calculated to be of 41.75 nm. The SeNPs after being formed will be stabilized by OC. Like other polysaccharides as alginate, dextran, gelatin, etc. OC has electron-rich functional groups such as –NH2, - OH groups that will stabilize SeNPs through coordinate bond and electrostatic repulsion. There are several factors that affect the stability of SeNPs solution such as H2SeO3 concentration, pH, stabilizer concentration, etc. [10, 11]. In particular, the temperature greatly affects the stability as well as the properties of SeNPs/OC solution The increasing size of SeNPs during storage time can be explained by increasing the Brownian motion when the SeNPs solution was stored at different temperatures. This results were also reported by Lin and Wang et al. [11]. From the above results, it can be seen that the appropriate temperature to store SeNPs/OC solution was 4 o C. However, SeNPs/OC solution is not always convenient to transport and apply. To overcome the limitation above as well as expand the scope of application, SeNPs in powder have been formed. Results from Fig 5 showed that SeNPs/OC powder prepared by spray drying technique from SeNPs/OC solution synthesized by gamma Co-60 ray irradiation was of high purity with composition only of SeNPs and OC. III. CONCLUSIONS SeNPs with concentration of 2.5 mM and diameter of ~42 nm stabilized in 2% OC solution were successfully synthesized by gamma Co-60 ray irradiation method. The appropriate temperature to store SeNPs/OC solution was 4 o C. SeNPs/OC powder with high purity was also prepared from SeNPs/dextran solution by spray drying technique. SeNPs/OC powder is potentially promising for use in injection or in oral administration for cancer therapy and for other purposes of application as well. REFERENCES [1]. H.W. Tan, H.Y. Mo, A.T.Y. Lau, Y.M. Xu. “Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy”, International Journal of Molecular Sciences, 20(1), 1-26, 2019. [2]. S. Skalickova, V. Milosavljevic, K. Cihalova, et al. “Selenium nanoparticles as a nutrition supplement”, Nutrition, 33, 83-90, 2017. [3]. J. Zhang, H. Wang, X. Yan, L. Zhang. “Comparison of short-term toxicity between nano-Se and selenite in mice”, Life Sciences, 76(10), 1099-1109, 2005. [4]. J. Zhang, X. Wang, T. Xu. “Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se- methylselenocysteine in mice”, Toxicological Sciences, 101(1), 22-31, 2008. [5]. X. Zhai, C. Zhang, G. Zhao, S. Stoll, F. Ren, X. Leng. “Antioxidant capacities of the selenium nanoparticles stabilized by chitosan”, Journal of nanobiotechnology, 15:4, 2017. [6]. P. Sonkusre, R. Nanduri, P. Gupta, S.S. Cameotra. “Improved extraction of intracellular biogenic selenium nanoparticles NGUYEN NGOC DUY et al. 31 and their specificity for cancer chemoprevention”, Journal of Nanomedicine & Nanotechnology, 5:2, 1000194, 2014. [7]. E.N. Ali, S.M. El-Sonbaty, F.M. Salem. “Evaluation of selenium nanoparticles as a potential chemopreventive agent against lung carcinoma”, International Journal of Pharmaceutical Biological and ChemicalSciences, 2(4), 38-46, 2013. [8]. E. Faghfuri, M.H. Yazdi, M. Mahdavi, Z. Sepehrizadeh, M.A. Faramarzi, F. Mavandadnejad, A.R. Shahverdi. “Dose- response relationship study of selenium nanoparticles as an immunostimulatory agent in cancer-bearing mice”, Archives of medical research, 46(1), 31-37, 2015. [9]. Y. Zhu, Y. Qian, H. Huang, M. Zhang. “Preparation of nanometer-size selenium powders of uniform particle size by γ- irradiation”, Materials Letters, 28(1-3), 119- 122, 1996. [10]. N.Q. Hien, P.D. Tuan, D.V. Phu, L.A. Quoc, N.T.K. Lan, N.N. Duy, T.T. Hoa. “Gamma Co-60 ray irradiation synthesis of dextran stabilized selenium nanoparticles and their antioxidant activity”, Materials Chemistry and Physics, 205, 29-34, 2018. [11]. Z.H. Lin, C.R.C. Wang. “Evidence on the size- dependent absorption spectral evolution of selenium nanoparticles”, Materials Chemistry and Physics, 92(2-3), 591-594, 2005.
File đính kèm:
 study_on_the_preparation_of_selenium_nanoparticles_by_gamma.pdf
study_on_the_preparation_of_selenium_nanoparticles_by_gamma.pdf

