Production of radioisotopes and radiopharmaceuticals at the Dalat nuclear research reactor
After reconstruction, the Dalat Nuclear Research Reactor (DNRR) was inaugurated on
March 20th, 1984 with the nominal power of 500 kW. Since then the production of radioisotopes and
labelled compounds for medical use was started. Up to now, DNRR is still the unique one in Vietnam.
The reactor has been operated safely and effectively with the total of about 37,800 hrs (approximately
1,300 hours per year). More than 90% of its operation time and over 80% of its irradiation capacity
have been exploited for research and production of radioisotopes. This paper gives an outline of the
radioisotope production programme using the DNRR. The production laboratory and facilities
including the nuclear reactor with its irradiation positions and characteristics, hot cells, production
lines and equipment for the production of Kits for labelling with 99mTc and for quality control, as well
as the production rate are mentioned. The methods used for production of 131I, 99mTc, 51Cr, 32P, etc.
and the procedures for preparation of radiopharmaceuticals are described briefly. Status of utilization
of domestic radioisotopes and radiopharmaceuticals in Vietnam is also reported.

Trang 1
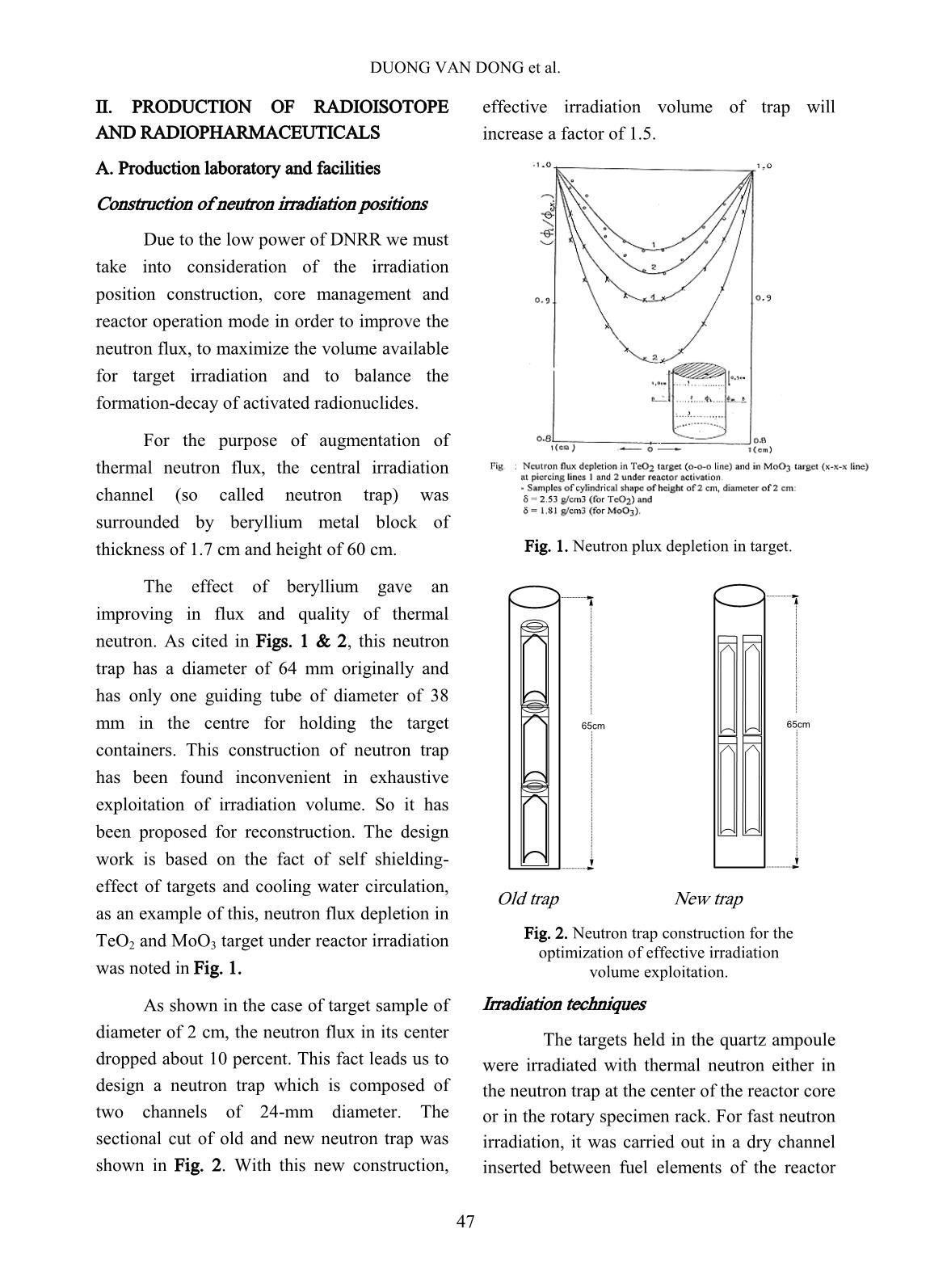
Trang 2
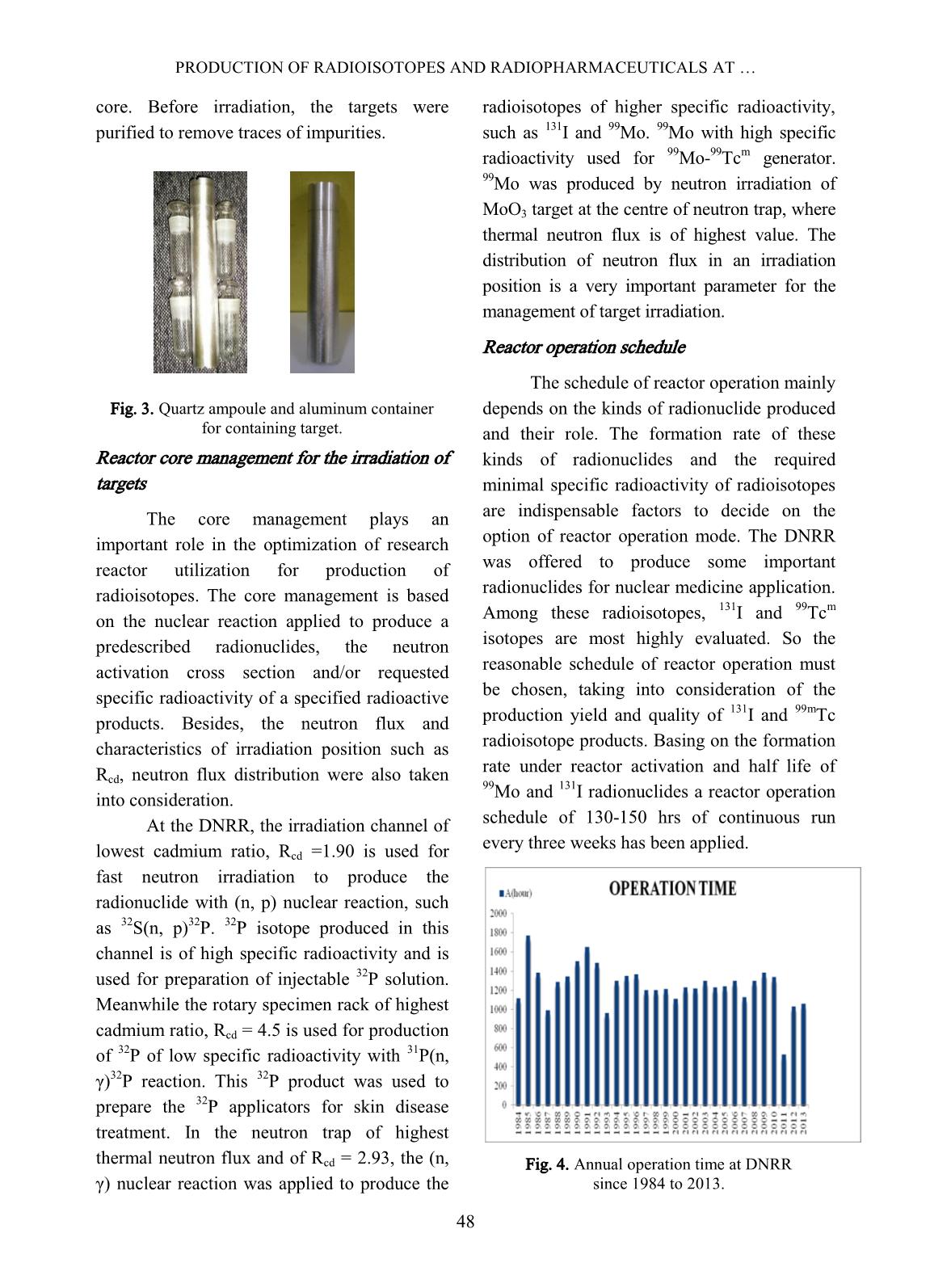
Trang 3

Trang 4

Trang 5
Trang 6
Trang 7
Trang 8

Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Production of radioisotopes and radiopharmaceuticals at the Dalat nuclear research reactor

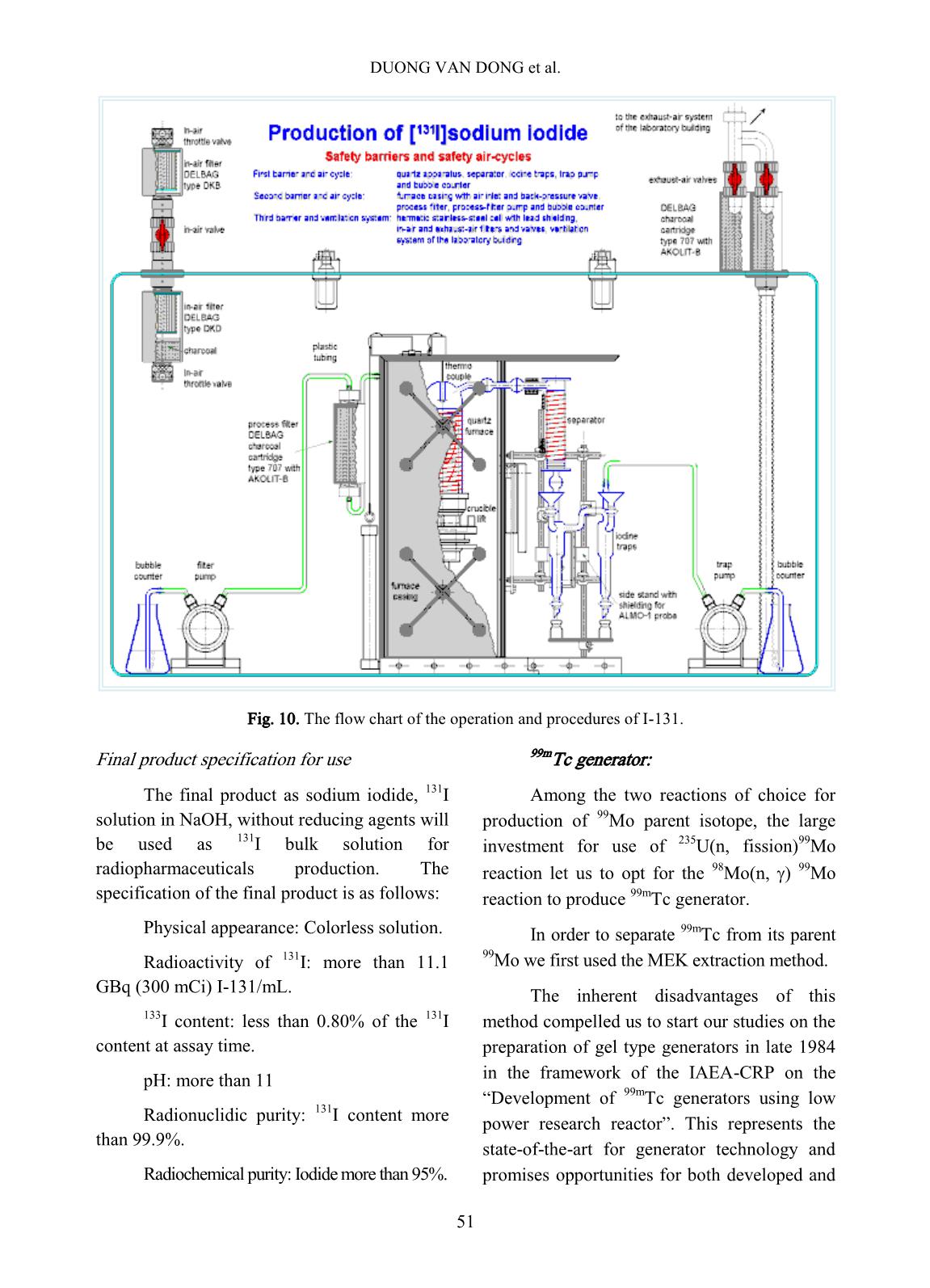
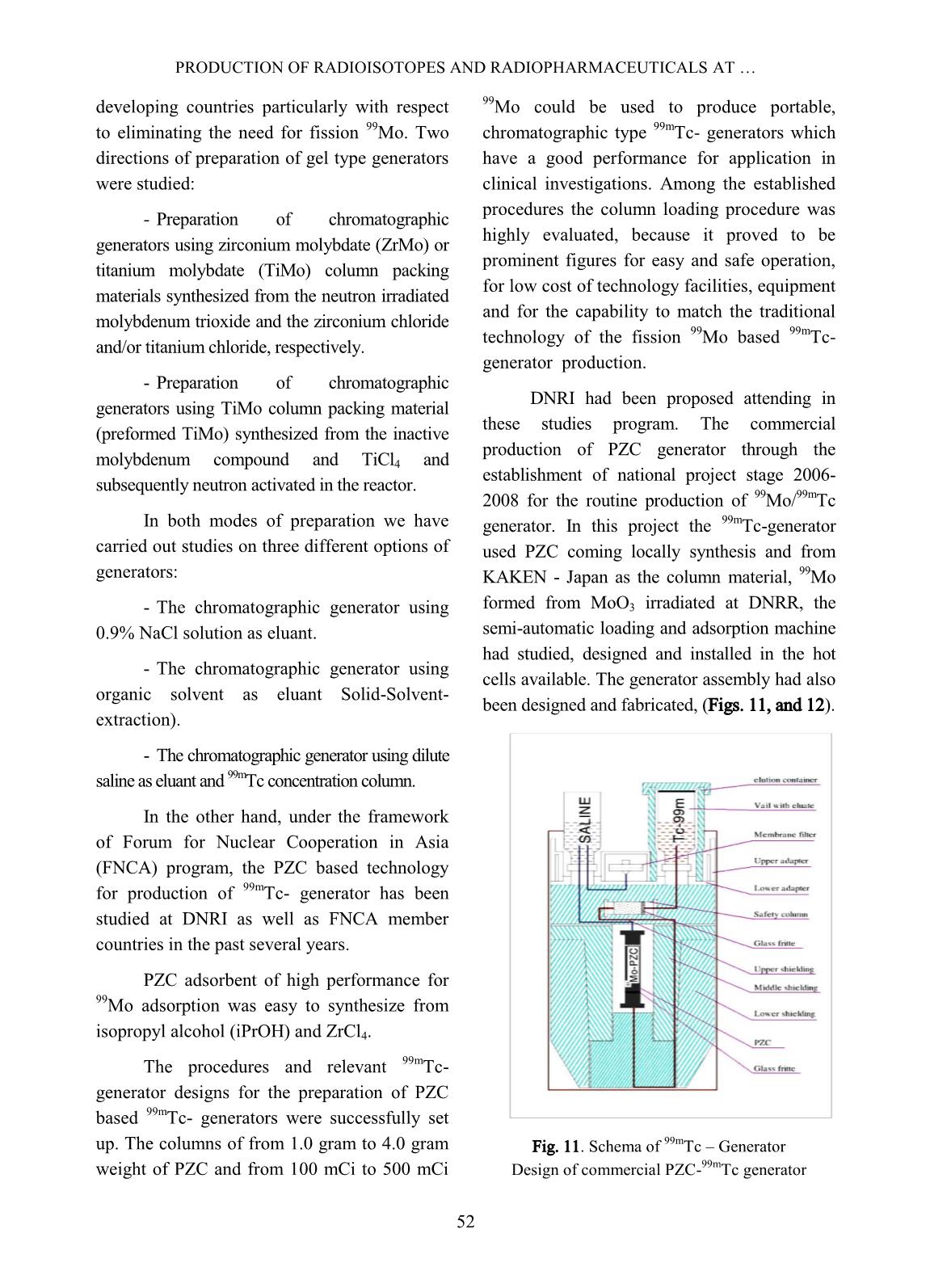
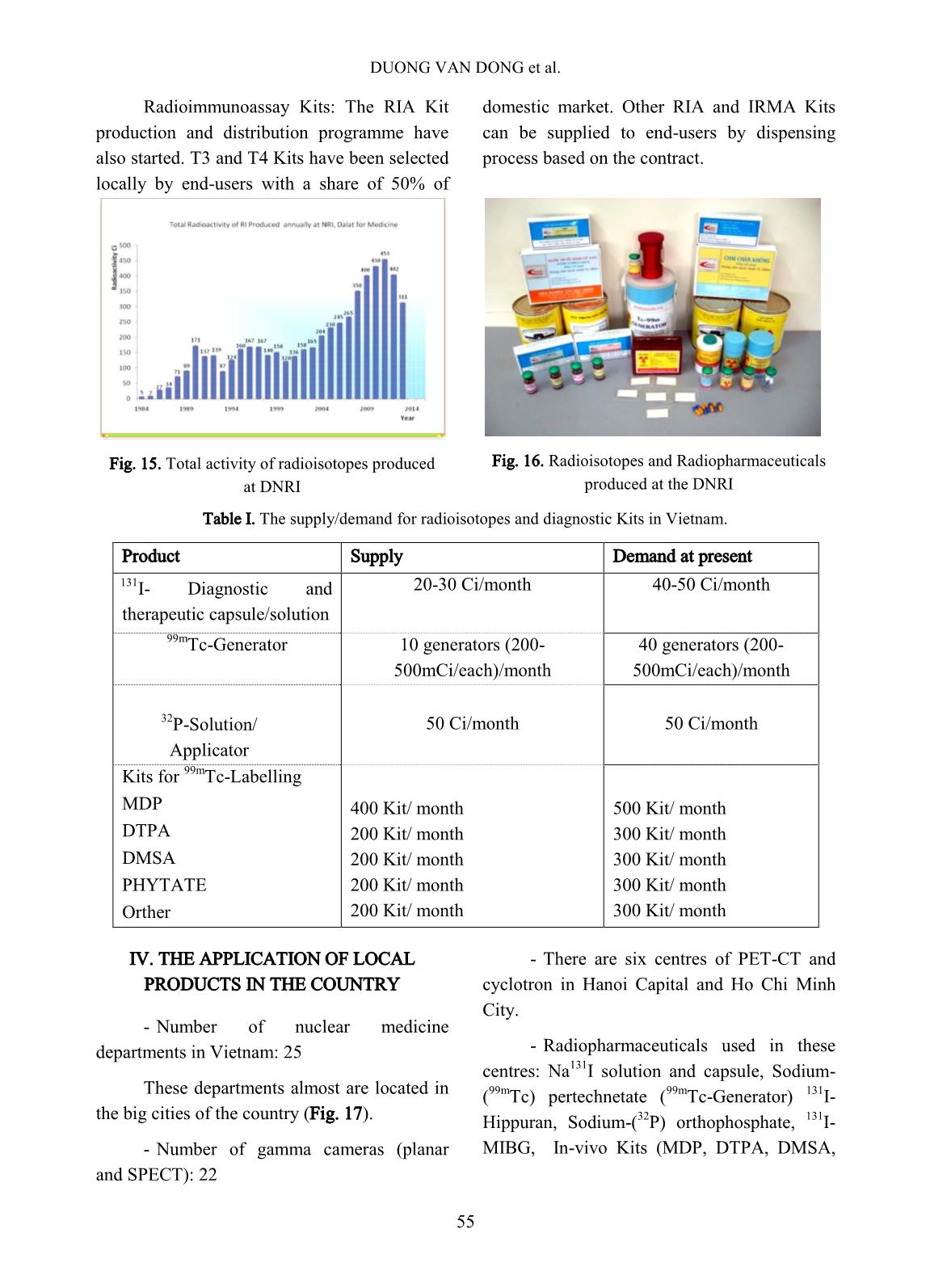
p. The target of tellurium dioxide contained in a welded aluminum capsule, according to the nuclear reaction as follows: The irradiated tellurium dioxide powder is transferred to a Vycor distillation vessel and connected to the iodine-131 tellurium processing system. The processing furnace is heated up to 750 o C in order to distill the iodine-131 over to a charcoal column trap connected in-line of the distillation system. The charcoal column trap is rinsed with the de- ionized water then eluted with sodium hydroxide 0.05N to form the final product of iodine-131 solution. The scheme in Fig. 10 shows the flow chart of the operation and procedures. The target used in the production is an analytical grade material of natural tellurium as tellurium dioxide obtained from Fluka Inc. The chemical purity of the target as TeO2 is >95%. The specification of the target before being fired in a muffle furnace through analysis by emission spectrograph should contain of selenium less than 0.05% and heavy metals less than 0.1%. After being fired in the muffle furnace the analysis should give selenium less than 0.005% and heavy metal less than 0.1%. DUONG VAN DONG et al. 51 Fig. 10. The flow chart of the operation and procedures of I-131. Final product specification for use The final product as sodium iodide, 131 I solution in NaOH, without reducing agents will be used as 131 I bulk solution for radiopharmaceuticals production. The specification of the final product is as follows: Physical appearance: Colorless solution. Radioactivity of 131 I: more than 11.1 GBq (300 mCi) I-131/mL. 133 I content: less than 0.80% of the 131 I content at assay time. pH: more than 11 Radionuclidic purity: 131 I content more than 99.9%. Radiochemical purity: Iodide more than 95%. 99mTc generator: Among the two reactions of choice for production of 99 Mo parent isotope, the large investment for use of 235 U(n, fission) 99 Mo reaction let us to opt for the 98 Mo(n, ) 99Mo reaction to produce 99m Tc generator. In order to separate 99m Tc from its parent 99 Mo we first used the MEK extraction method. The inherent disadvantages of this method compelled us to start our studies on the preparation of gel type generators in late 1984 in the framework of the IAEA-CRP on the “Development of 99mTc generators using low power research reactor”. This represents the state-of-the-art for generator technology and promises opportunities for both developed and PRODUCTION OF RADIOISOTOPES AND RADIOPHARMACEUTICALS AT 52 developing countries particularly with respect to eliminating the need for fission 99 Mo. Two directions of preparation of gel type generators were studied: - Preparation of chromatographic generators using zirconium molybdate (ZrMo) or titanium molybdate (TiMo) column packing materials synthesized from the neutron irradiated molybdenum trioxide and the zirconium chloride and/or titanium chloride, respectively. - Preparation of chromatographic generators using TiMo column packing material (preformed TiMo) synthesized from the inactive molybdenum compound and TiCl4 and subsequently neutron activated in the reactor. In both modes of preparation we have carried out studies on three different options of generators: - The chromatographic generator using 0.9% NaCl solution as eluant. - The chromatographic generator using organic solvent as eluant Solid-Solvent- extraction). - The chromatographic generator using dilute saline as eluant and 99m Tc concentration column. In the other hand, under the framework of Forum for Nuclear Cooperation in Asia (FNCA) program, the PZC based technology for production of 99m Tc- generator has been studied at DNRI as well as FNCA member countries in the past several years. PZC adsorbent of high performance for 99 Mo adsorption was easy to synthesize from isopropyl alcohol (iPrOH) and ZrCl4. The procedures and relevant 99m Tc- generator designs for the preparation of PZC based 99m Tc- generators were successfully set up. The columns of from 1.0 gram to 4.0 gram weight of PZC and from 100 mCi to 500 mCi 99 Mo could be used to produce portable, chromatographic type 99m Tc- generators which have a good performance for application in clinical investigations. Among the established procedures the column loading procedure was highly evaluated, because it proved to be prominent figures for easy and safe operation, for low cost of technology facilities, equipment and for the capability to match the traditional technology of the fission 99 Mo based 99m Tc- generator production. DNRI had been proposed attending in these studies program. The commercial production of PZC generator through the establishment of national project stage 2006- 2008 for the routine production of 99 Mo/ 99m Tc generator. In this project the 99m Tc-generator used PZC coming locally synthesis and from KAKEN - Japan as the column material, 99 Mo formed from MoO3 irradiated at DNRR, the semi-automatic loading and adsorption machine had studied, designed and installed in the hot cells available. The generator assembly had also been designed and fabricated, (Figs. 11, and 12). Fig. 11. Schema of 99m Tc – Generator Design of commercial PZC- 99m Tc generator DUONG VAN DONG et al. 53 Fig. 12. The semi-automatic loading machine In conclusion, it is strongly believed that ZrMo, TiMo and PZC based generator play an importance role as alternative technology for production of 99 Mo/ 99m Tc generator from reaction 98Mo(n, γ)99Mo. However these methods were not very appropriate for the low power research reactor as DNRR. Because of those reasons, it is necessary to build a new research reactor with power at least of 10 MW, and the neutron flux is high enough to research and produce radioisotopes. Phosphorus-32: 32 P isotope was produced according to two nuclear reactions: 32 S(n, p) 32 P and 31 P(n, )32P. The first reaction was used for the production of injectable carrier-free 32 P solution, the second for that of 32 P –isotope applicators for skin disease treatment. First the injectable 32 P solution of radioactivity of ten mCi scale was produced from irradiated MgSO4 target using magnesia as absorbent to separate 32 P isotope from MgSO4 solution. In the case of Ci scale production, the large amount of waste produced from this technology caused storage problems. Recently, we have introduced the distillation technique to separate 32 P from reactor irradiated elemental sulfur. Our glass apparatus for this production process is shown in Fig. 13. It can be used for distillation either in the vacuum or in the N2 gas flow by changing the upper stopper of the distillation vessel. The distillation parameters and post-distillation purification of 32 P solution were adopted as described in literature. Fig. 13. The glass apparatus for 32 P production process using nuclear reaction 32 S(n, p) 32 P The 32 P applicators for skin disease treatment were produced by neutron irradiation of a soft plate preformed from cloth binder and a covering mixture of red phosphorus and glue. After irradiation in the reactor, the radioactive plate was impregnated with plastic and covered with Scotch adhesive. The mechanical strength of the preformed plate was not lost under 75-hour irradiation in a thermal neutron flux of 5x10 12 n.cm -2 .s -1 . Under this irradiation a plate containing 65 mg P per square centimeter gives a radioactivity of 15 mCi 32 P. The absorbed dose rate on the surface of the plate of size 50 x 40 mm 2 was measured as 110 Rad.min -1 at the center and 75 Rad.min -1 on the edge. Medical doctors’ experience over ten years showed that with repeated treatment of three or five 15-minute applications the following diseases will be cured: Eczema, skin cancer, bump scar, etc. At present more than 75 Ci 32 P in applicator form are used annually in the country. PRODUCTION OF RADIOISOTOPES AND RADIOPHARMACEUTICALS AT 54 Cr-51 isotope: The production of 51 Cr isotope was carried out based on the Szilard-Charmel reaction using reagent grade K2CrO4 target. The chemical separation of recoiled 51 Cr nuclide was based on the selective adsorption of this isotope on an inorganic ion exchanger Si-ZrP (Silica gel supported zirconium - phosphate) synthesized by us. Other isotopes were also produced when requested in small amounts for industrial and agricultural applications. The methods for production of these isotopes were selected from investigation results or different reference sources. D. Production of Kits for labelling with 99m Tc: In furthering the application of 99m Tc isotope, the local availability of Kits for labelling with 99m Tc plays an important role. With IAEA support the basic equipment for the production of Kits has been installed in our laboratory. At present many kinds of in-vivo Kits have been successfully prepared and put to use in the country, they are Phytate, Gluconate, Pyrophosphate, Citrate, DMSA, HIDA, DTPA, Maccroaggregated HSA and EHDP (1-hydroxy ethylidene-1, 1-disodium- phosphate). The studies on the preparation of Radioimmuno-assay Kits and therapeutic agents and/or radionuclides were also carried out. The future production of the above mentioned items is foreseen and planned. E. Quality control Radioisotope and radiopharmaceutical quality control was carried out for all batches of our products. The gamma spectrum analysis using Ge-Li detector coupled with a multichannel analyser is used for radionuclide purity control, the TLC, HPLC and gel- chromatography techniques for chemical purity, and the spectrometry and neutron activation analysis for chemical purity. Biodistribution assay, biological tests (apyrogenity, sterility, toxicity) and physicochemical tests (pH, turbidity) are also carried out regularly. Fig. 14. HPLC system for QC. III. LOCAL PRODUCTION VOLUME AND DEMAND These types of radioisotopes have regularly been supplied to more than 25 hospitals in Vietnam two times per month. The 131 1 radioisotope labelled radiopharmaceuticals such as 131 I-Hippuran; 131 IMIBG have also been regularly supplied to hospitals. Radioisotope production rate is shown in Fig. 15 and Table I. In order to support the application of 99m Tc, 113m In, 177 Lu and 153 Sm radioisotopes in clinical diagnosis and therapeutics, the preparation of radiopharmaceuticals in Kit forms has been carried out. The following Kits have regularly been manufactured in DNRI: Phytate, Gluconate, Pyrophosphate, Citrate, DMSA, EHIDA, DTPA, HSA macroaggregated, HEDP, HmPAO, MIBI, MDP. DUONG VAN DONG et al. 55 Radioimmunoassay Kits: The RIA Kit production and distribution programme have also started. T3 and T4 Kits have been selected locally by end-users with a share of 50% of domestic market. Other RIA and IRMA Kits can be supplied to end-users by dispensing process based on the contract. Fig. 15. Total activity of radioisotopes produced at DNRI Fig. 16. Radioisotopes and Radiopharmaceuticals produced at the DNRI Table I. The supply/demand for radioisotopes and diagnostic Kits in Vietnam. Product Supply Demand at present 131 I- Diagnostic and therapeutic capsule/solution 20-30 Ci/month 40-50 Ci/month 99m Tc-Generator 10 generators (200- 500mCi/each)/month 40 generators (200- 500mCi/each)/month 32 P-Solution/ Applicator 50 Ci/month 50 Ci/month Kits for 99m Tc-Labelling - MDP - DTPA - DMSA - PHYTATE - Orther 400 Kit/ month 200 Kit/ month 200 Kit/ month 200 Kit/ month 200 Kit/ month 500 Kit/ month 300 Kit/ month 300 Kit/ month 300 Kit/ month 300 Kit/ month IV. THE APPLICATION OF LOCAL PRODUCTS IN THE COUNTRY - Number of nuclear medicine departments in Vietnam: 25 These departments almost are located in the big cities of the country (Fig. 17). - Number of gamma cameras (planar and SPECT): 22 - There are six centres of PET-CT and cyclotron in Hanoi Capital and Ho Chi Minh City. - Radiopharmaceuticals used in these centres: Na 131 I solution and capsule, Sodium- ( 99m Tc) pertechnetate ( 99m Tc-Generator) 131 I- Hippuran, Sodium-( 32 P) orthophosphate, 131 I- MIBG, In-vivo Kits (MDP, DTPA, DMSA, PRODUCTION OF RADIOISOTOPES AND RADIOPHARMACEUTICALS AT 56 Phosphon, Glucon, Phytate, MAHSA, EHIDA, HMPAO, MIBI, MAG-3, etc.). - Locally manufactured products take about 50% of total market. In order to get a higher market share now we increase the production by loading generations with importing raw materials such as 99 Mo and 131 I solutions. Fig. 17. Location of Nuclear Medicine Departments in Vietnam. REFERENCES [1] Le Van So, Production of 99mTc isotope from the chromatographic generator using zirconium- molydate and titanium-molybdate targets as column packing materials. Research Co- ordination Meeting, Bandung, Indonesia, (October 1987). [2] Radioisotope production and quality control. Technical Reports Series No. 128. IAFA, Vienna, (1971). [3] Le Van So, Investigation on the silica gel supported form of micro crystalline zirconium- phosphate ion exchanger and its applications in chemical separation. I.- Preparation, ion exchange properties and stability of Si-ZrP, J. Radioanal. Nucl. Chem., (Articles) 9 (1) 17-30 (1986). [4] Le Van So, Richard M. Lambrecht, Development of alternative technologies for a gel-type chromatographic 99mTc generator. J. Labelled Compd. Radiopharm. 35:270 (1994). [5] Ngo Quang Huy et al, Reactor physics experimental studies on Dalat nuclear research reactor, 50A-01-04 Research Project Final Report, (1990) (in Vietnamese). [6] Tran Ha Anh et al, Studies on Dalat Nuclear Reactor Physics and Technique and on Measures to ensure the safety and efficiency of the reactor, KC-09-15 Research Project Final Report, (1995). [7] Nguyen Nhi Dien, Dalat nuclear research reactor - status of operation and utilization, Dalat Sym. -RR-PI-05, Dalat, (2005). [8] Duong Van Dong, Status of Radioisotope Production and Application in Vietnam, Dalat Sym. -RR-PI-09, Dalat, (2009). [9] Duong Van Dong, Status of the study on PZC based Tc-99m generator and potential of its commercial production in Vietnam, Nihon Genshiryoku Kenkyu Kaihatsu Kiko JAEA- Conf, Journal Code: L2150A, page 25-29 (2007).
File đính kèm:
 production_of_radioisotopes_and_radiopharmaceuticals_at_the.pdf
production_of_radioisotopes_and_radiopharmaceuticals_at_the.pdf

