Nuclear Science and Technology - Volume 8, Number 4, December 2018
Reactivity induced transient analysis when the occurrence
of leakage in the dry irradiation channels
of the Dalat Nuclear Research Reactor
Dau Duc Tu, Nguyen Minh Tuan, Le Vinh Vinh, Huynh Ton Nghiem,
Nguyen Kien Cuong, Tran Quoc Duong, Bui Phuong Nam
Reactor Center, Dalat Nuclear Research Institute, VINATOM
01 Nguyen Tu Luc Str., Dalat, Lamdong, Vietnam.
E-mail: tudd.re@dnri.vn
(Received 06 December 2018, accepted 28 December 2018)
Abstract: The leakage from the reactor pool back into the dry irradiation channels due to corrosion or
mechanics based reason is a postulated event that could occur under operating conditions of the Dalat
nuclear research reactor (DNRR), especially the channel 7-1 which has been installed more than 30
years. When it occurs, the air space in these channels will be occupied by the water, subsequently a
water column will appear in fuel region. The appearance of water column considerably enhances
medium of neutron moderation for its surrounding fuel assemblies. As a result, a positive reactivity is
inserted in the core and this event is classified as an insertion of excess reactivity. This event needs to
be addressed by analysis and assessment from safety point of view and the results of analysis are also
important for updating the reactor operating procedures. This paper presents assumptions, computer
models and the results of analysis for such event in the DNRR by using MCNP5 code (code for
neutronics analysis) and EUREKA-2/RR code (code for transient analysis). The calculation results
include value of reactivity insertion, change in power of reactor, as well as surface temperature of the
hottest fuel assembly. This research contributes to updating the reactor operating procedure.
Keywords: Dalat nuclear research reactor (DNRR), MCNP5, EUREKA-2/RR, reactivity
insertion, transient analysis.
I. INTRODUCTION
A. General information
The Dalat reactor, which is a swimming
pool type reactor and uses light water as
moderator and coolant, has 500 kW thermal
power and put into operation in March, 1984. It
was reconstructed and upgraded from the USA
made 250 kW TRIGA reactor. A number of
structures from the original TRIGA reactor such
as the aluminum tank with the surrounding
concrete shield, four beam-ports, thermal
column and the graphite reflector were
remained.
The reactor core is currently loaded 92
low enriched fuel (LEU) assemblies (19.75%),
3 irradiation channels (2 dry channels and 1
wet channel), 12 beryllium rods and a neutron
trap [1]. The fuel assemblies are VVR-M2
tubular fuel assemblies designed
and manufactured in Russia. Each fuel
assembly consists of three coaxial annular
tubes as well as a header and a tail. The
outermost fuel element has a hexagonal shape
of 32 mm in width across parallel sides and the
other two inner ones have a circular shape of
22 mm and 11 mm in outer diameter,
respectively. The beryllium rods of the reactor
have a hexagonal shape of 32 mm in width
across its parallel sides [1].

Trang 1
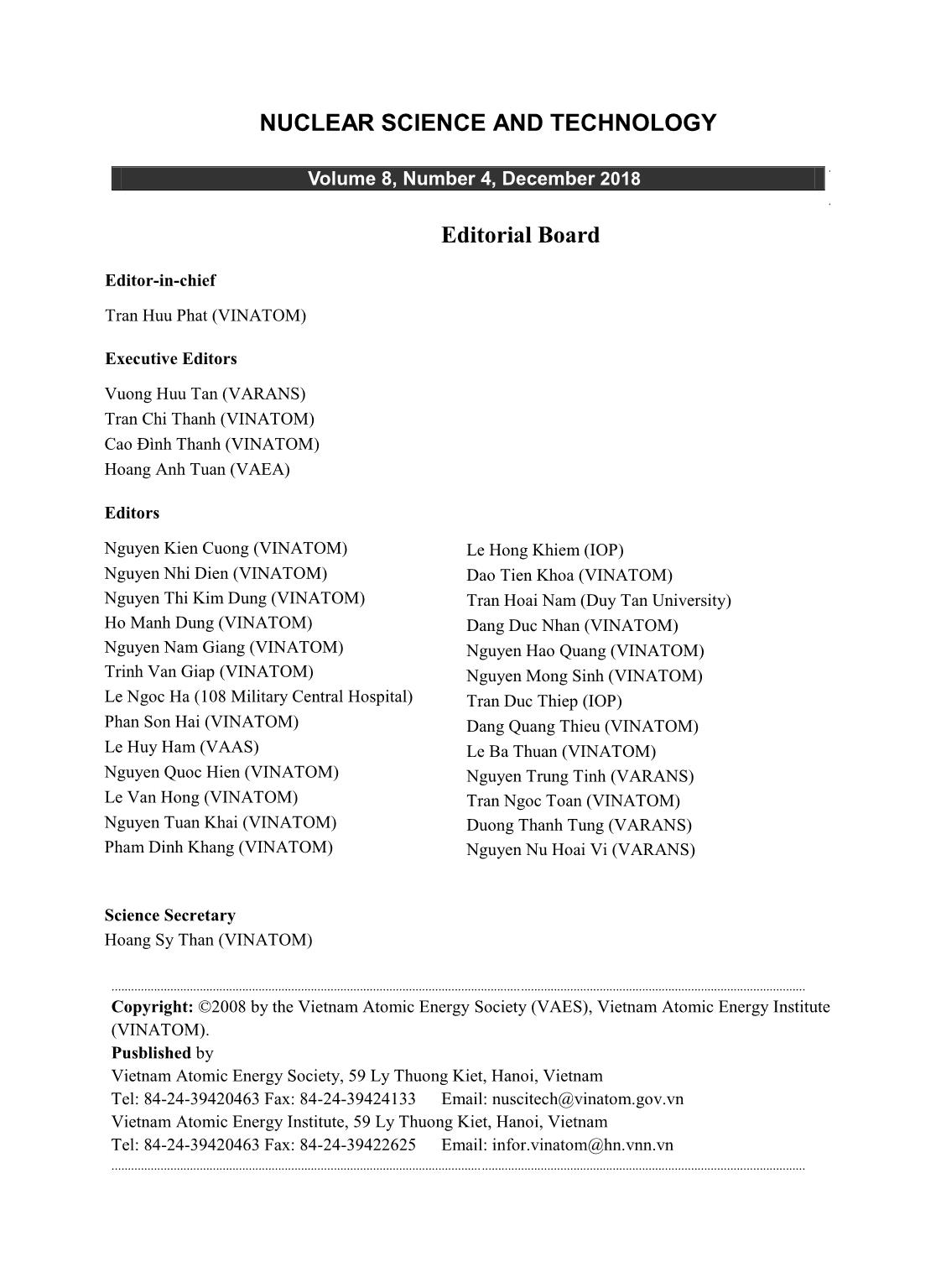
Trang 2

Trang 3

Trang 4
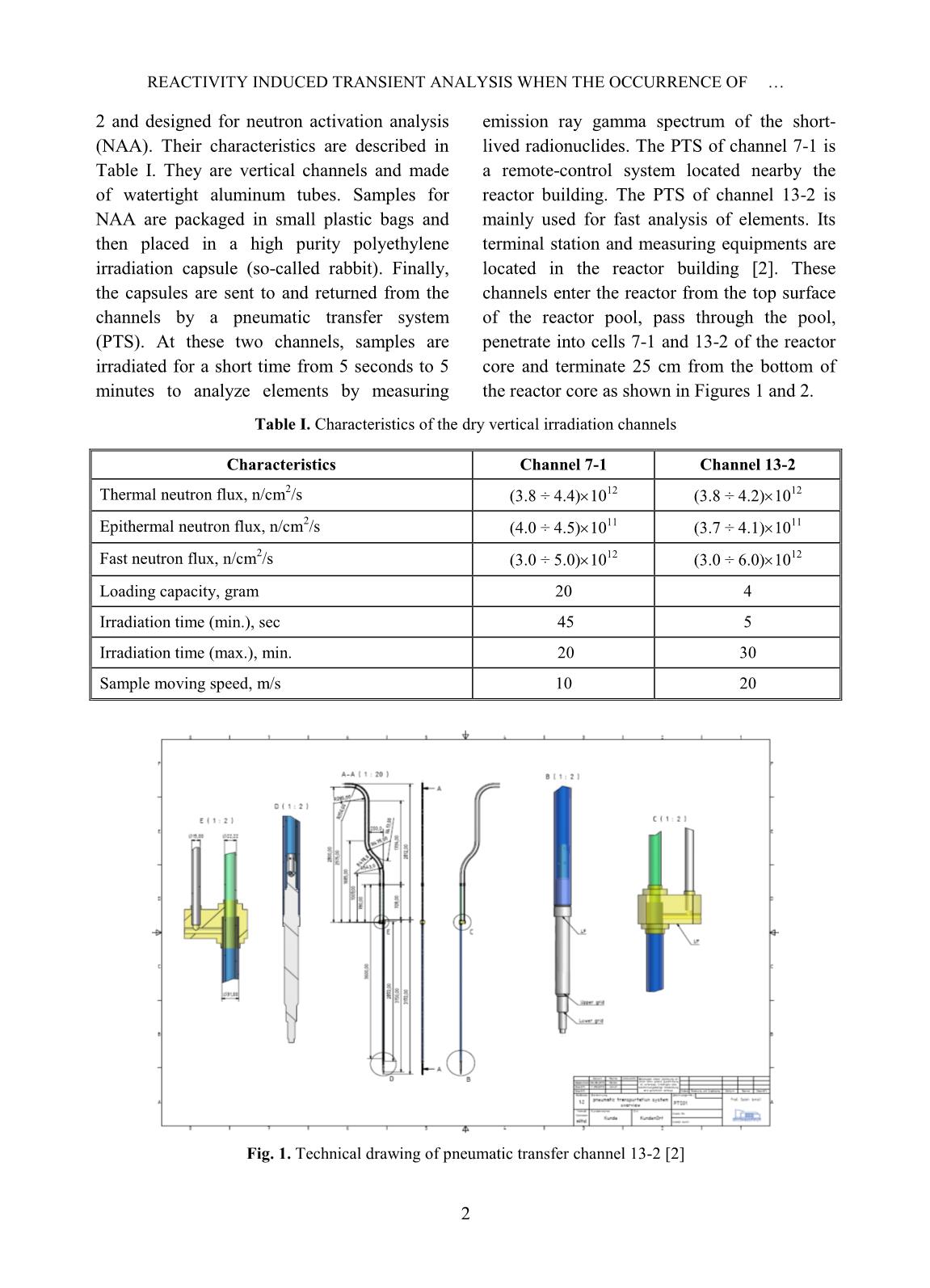
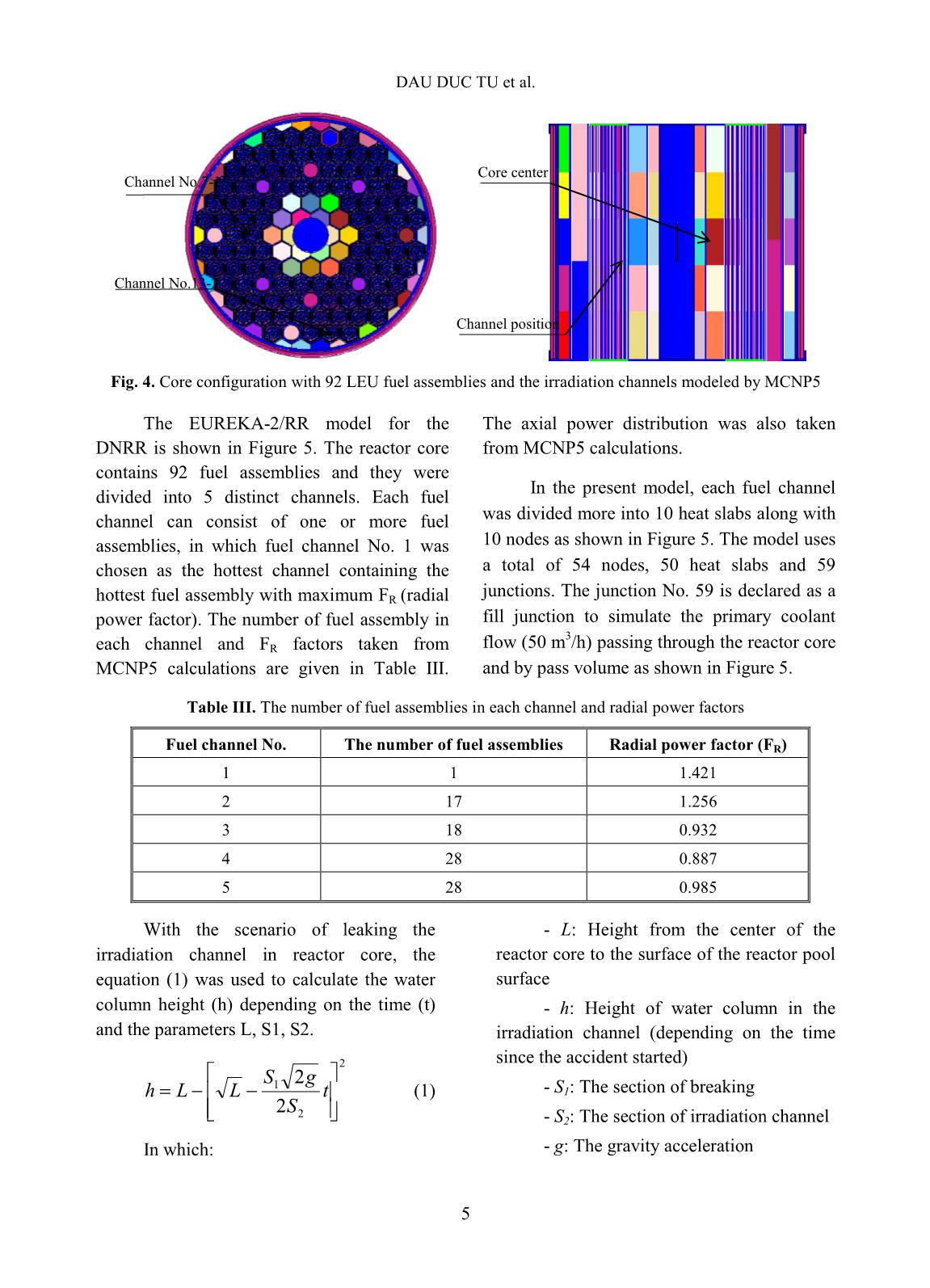
Trang 5
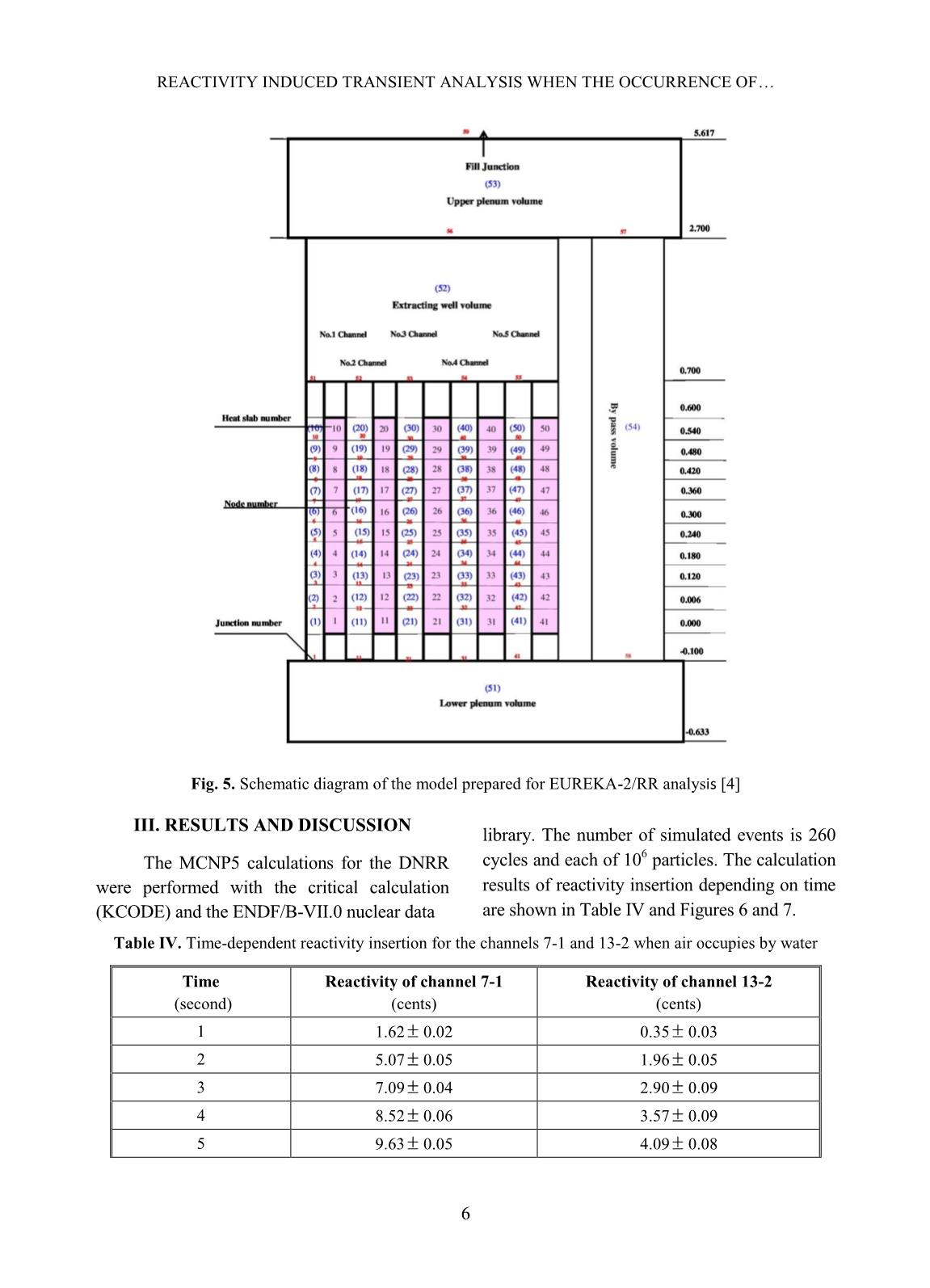
Trang 6
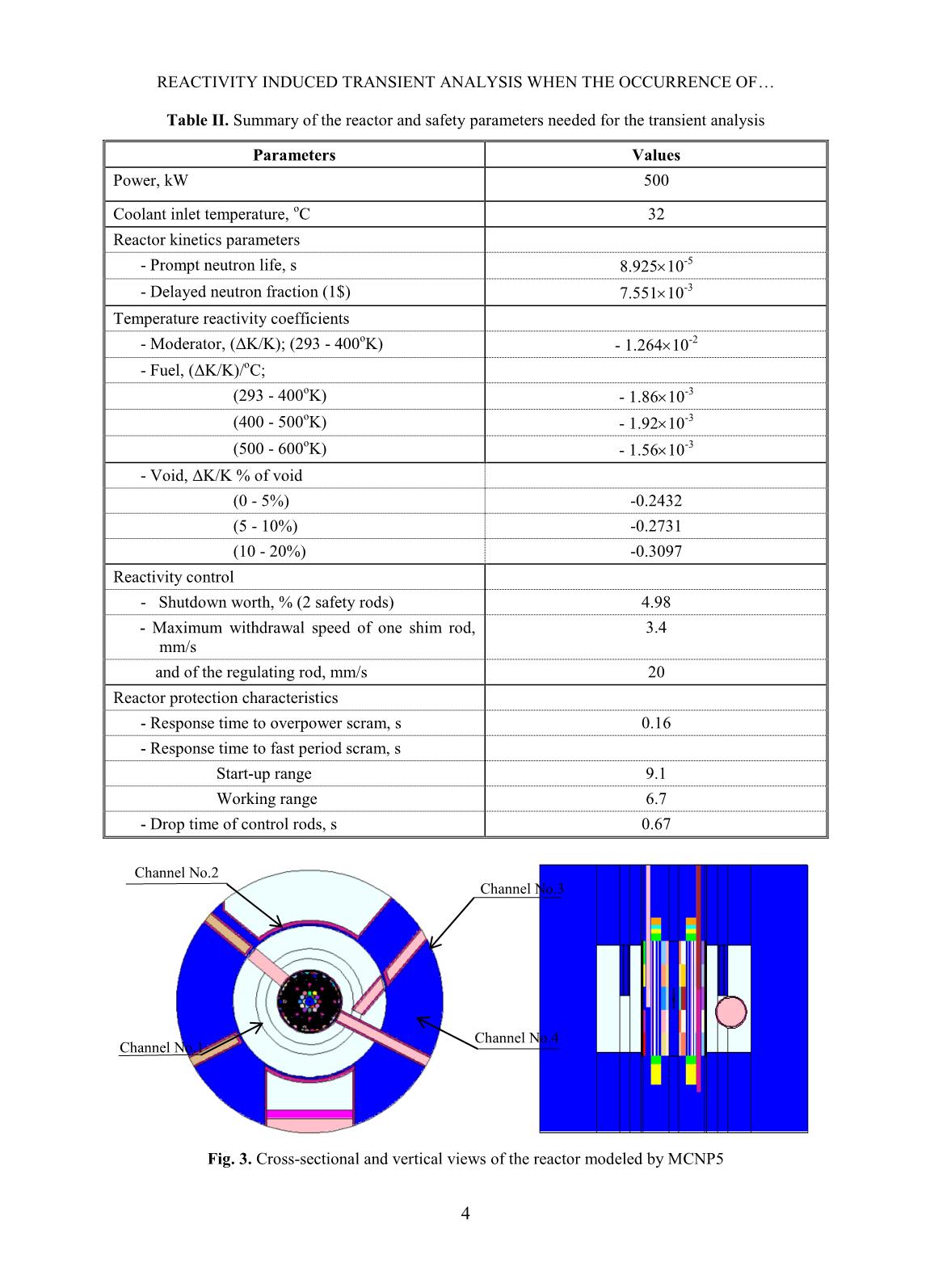
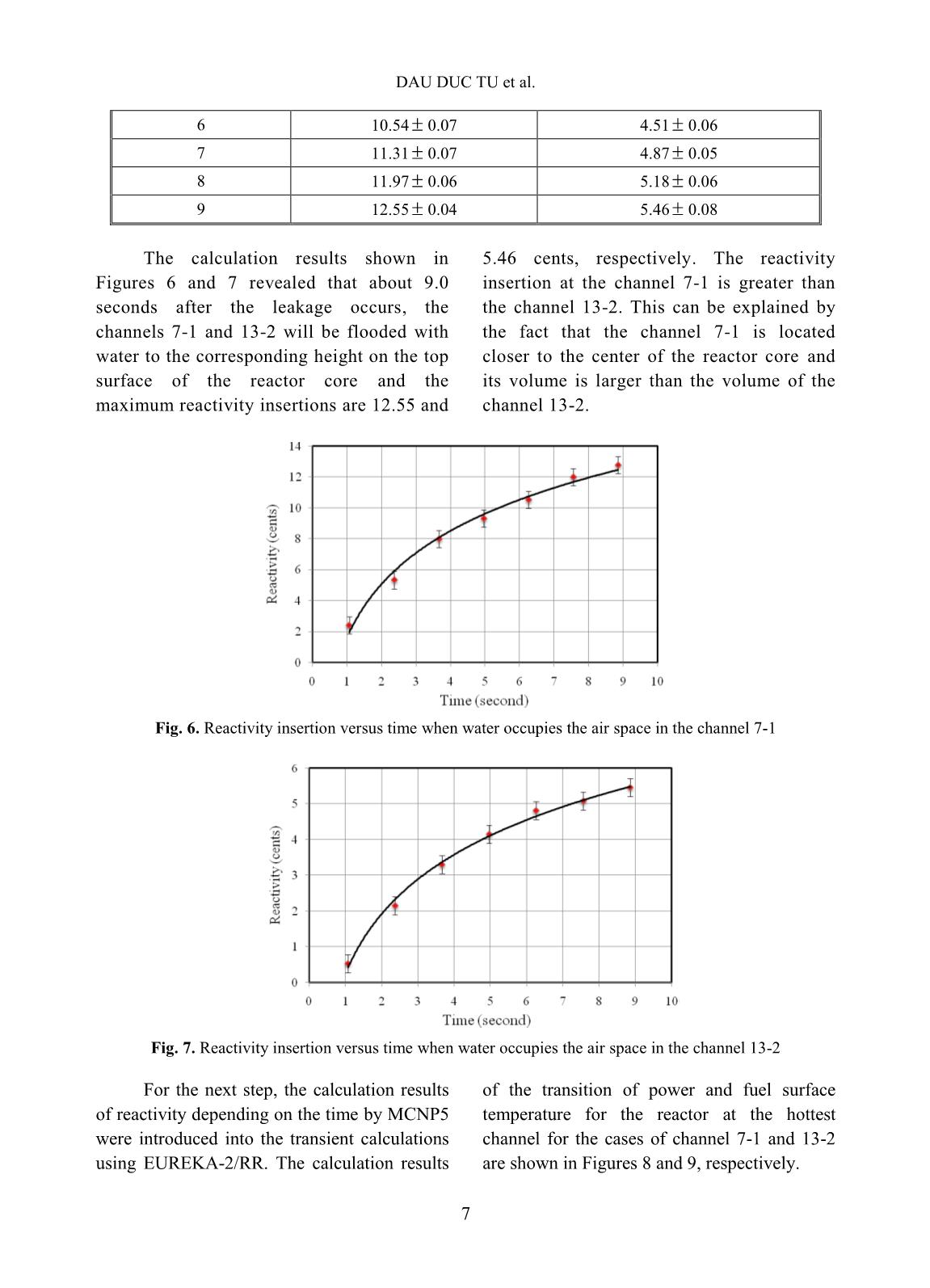
Trang 7
Trang 8
Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Nuclear Science and Technology - Volume 8, Number 4, December 2018

obaric interference caused by Rb on the determination of strontium isotope ratio. However, the small amount of calcium remained in sample solution at this stage was also considered when using cation chromatography technique for removal of Rb interference. The mixed solution containing 1.5 ppm Ca, 0.5 ppm Sr and 0.25 ppm Rb was loaded on the cation exchanger column. The elution was carried out under the same condition as above mentioned. The elution curve is shown in Fig. 7. Fig.7 Elution curves of Rb, Ca and Sr under gradient conditions STUDY ON THE REMOVAL OF INTERFERENCES FOR THE DETERMINATION OF 87 Sr/ 86 Sr 40 The elution peaks from Fig.7 depict that almost Ca presented in mixed solution was eluted together with Sr meanwhile Rb was completely removed within the first 7 eluted fractions. It confirms that the small amount of Ca in sample solution does not interfere with the quantitative separation of Rb, and that the isobaric effect caused by Rb can be completely removed. C. Validation of separation procedure The recovery of strontium through separation procedure was studied by using Sr isotopic standard solutions, in which total concentration of strontium was fixed as 100 μg/L but the isotope ratio 86Sr/87Sr was various with the addition of a certain amount of 86 Sr isotope standard solution in to the natural Sr standard solution (see Table II) in a matrix (100 mg/L Ca and 50 μg/L Rb). The separation procedure was repeated for all synthesized samples under the same conditions as reported above. The results were given in Table II. Table II. Recovery of Sr in synthesized samples Total Sr (µg/L) Spiked 86 Sr (µg/L) 86 Sr/ 87 Sr (by theory) 87 Sr found (µg/L) Total Sr found (µg/L) Recovery (%) 100 0 1.40857 7.80047 103.605 103.61 90 10 2.98187 6.35607 90.801 100.89 80 20 4.90220 5.72470 81.781 102.22 60 40 10.4921 4.40357 62.908 104.85 40 60 21.9123 2.92630 41.804 104.51 The data in Table II show that the recovery of strontium for whole cases (from 100.89% to 104.85%) seemed reliable for Sr analysis through the long procedure of mutual separation. It is thus suitable for the application of Sr isotopic ratio analysis in petroleum drill- holes water samples. The accuracy of separation method was studied by the use of NIST SRM 987 (SrCO3) isotopic standard reference material. The isotopic standard solution was prepared by dissolving a certain amount of standard reference material in dilute HNO3 and a small portion of this solution containing 100 μg/L (as total Sr concentration) was loaded on anion exchanger column. Whole separation procedure was carried out for this standard sample and the final elution fractions were collected for the determination of 87 Sr/ 86 Sr isotopic ratio on ICP-MS. Five replicates of experiment were done and the results were given in Table III. Table III. Analysis of the standard sample SRM 987 87 Sr/ 86 Sr certified value 87 Sr/ 86 Sr analyzed value Absolute Error (%) 0,71034 ± 0.00026 0.71453 ± 0.00836 + 0.59 The relative correctness of analyzed value is 99.41% to that of the certified value for NIST SRM 987, which seems reasonable in NGUYEN THI KIM DUNG, THAI THI THU THUY 41 this study due to the contribution of signal measurement deviation of instrument to the error. That is also the reason why the more precision of the isotopic analysis can be obtained from MC-ICPMS [1]. D. Analysis of petroleum drill-holes water samples Petroleum drill-holes water samples were pretreated to remove the oil and suspended solid particles as denoted in procedure. The chemical composition of sample was analyzed using ICP-MS in order to preliminary classification of the solution matrix. The sample solution was diluted with pure water as needed before applying the separation procedure, followed by the isotopic ratio measurement on ICP-MS. The analytical data were given in Table IV together with total concentrations of Rb and Sr and relative standard deviation (RSD) of 87 Sr/ 86 Sr isotopic ratio measurement. Table IV. Analysis of petroleum drill-holes water samples Sample Code Total Rb (µg/L) Total Sr (µg/L) 87 Sr/ 86 Sr Isotopic ratio Value RSD (%) EW02 36.6 989.8 0.70715 1.17 EW05 45.3 3502.5 0.70734 2.78 EW17 34.5 1240.1 0.70699 1.52 PW03 80.0 824.0 0.70686 1.45 PW04 60.2 423.5 0.70639 1.63 PW14 120.0 1980.0 0.70674 2.46 Analytical results showed that, 87 Sr/86Sr isotopic ratio of petroleum drill-holes water samples was various with different sample matrix. These data relatively agreed with those, which were obtained from similar study [4] of drill-holes water in Vietnam Petroleum Institute, where the water samples were pretreated and the analysis of 87 Sr/ 86 Sr isotopic was carried out by TIMS in over-sea laboratory. IV. CONCLUSIONS The removal of calcium in matrix from petroleum drill-holes water samples and the elimination of rubidium isobaric interference with strontium isotopic ratio determination were successfully achieved by using ion exchange chromatography. The anion exchange resin (Bio-Rad AG1X8 200-400 mesh) was employed for the separation of major calcium by 0.25 M HNO3 in 95% methanol with Sr recovery over 99%. The mutual separation of rubidium and strontium by gradient conditions of HNO3 concentration and flow rate on cation exchanger (Bio-Rad AG50X8 200-400 mesh) was taken part with nearly complete Sr recovery. The validation of method was also studied using isotopic standard solution and standard reference material with relative correctness of the analyzed value about 99.41% to the certified value of NIST SRM 987 reference material. The analytical procedure was then applied for the determination of 87 Sr/86Sr isotopic ratio in STUDY ON THE REMOVAL OF INTERFERENCES FOR THE DETERMINATION OF 87 Sr/ 86 Sr 42 petroleum drill-holes water samples using ICP- MS, which would contribute to the development of an analytical method to supply the demand of petroleum research and exploitation in Vietnam. ACKNOWLEDGEMENT The authors are thankful to the assistance of M.Sc. Ngo Quang Huy on carrying out some preliminary experiments. The financial support under framework of a VINATOM project encoded DTCB.09/18/VCNXH was highly appreciated. REFERENCES [1]. S.Garcia-Ruiz, M.Moldovan and J.I.G.Alonso, "Measurement of strontium isotope ratios by MC-ICP-MS after online Rb-Sr ion chromatography separation", J.Anal.At.Spectrom., 23, 84-93, 2008. [2]. S. Chassery, F. E. Grousset, G. Lavaux, and C. R. Quetel, “87Sr/86Sr measurements on marine sediments by inductively coupled plasma-mass spectrometry,” Fresenius J. Anal. Chem., Vol. 360, 230–234, 1998. [3]. M. E. dos Santos, H. E. L. Palmieri, and R. M. Moreira, “Testing the 87Sr/ 86Sr isotopic ratio measured by ICP-MS as a tracer for inter-well investigation in oil reservoirs,” EPJ Web of Conferences, 50, p. 02004, 2013. [4]. Luong Van Huan, Le Thi Thu Huong, Hoang Long, Nguyen Minh Quy, Vo Thi Tuong Hanh, “Application of stable isotope (δ18O and δ2H) analysis and 87Sr/86Sr equilibrium to determine the source of produced water in the oil field”, Petrovietnam Journal, No.4, pp. 24-31 (in Vietnamese), 2014. [5]. Richard J. Howarth and John M. McArthur, “Statistics for Strontium Isotope Stratigraphy: A Robust LOWESS Fit to the Marine Sr- Isotope Curve for 0 to 206 Ma, with Look-up Table for Derivation of Numeric Age”, The Journal of Geology, Volume 105, p. 441–456, 1997. [6]. K. Notsu, H. Wakita, and Y. Nakamura, “Strontium isotopic composition of oil-field and gas-field waters, Japan”, Appl. Geochemistry, Vol. 3, No.2, 173–176, 1988. [7]. Toshiro Takahashi, Yuka hirahara, Takashi Miyazaki, Bogdan stefanov Vaglarov, Qing Chang, Jun-ichi Kimura, Yoshiyuki Tatsumi, “Precise determination of Sr isotope ratios in igneous rock samples ans application to micro- analysis of plagioclase phenocrysts”, JAMSTEC-R IFREE Special Issue, 59-64, Nov. 2009. [8]. K. Ariyama, M. Shinozaki, and A. Kawasaki, “Determination of the geographic origin of rice by chemometrics with strontium and lead isotope ratios and multielement concentrations”, J. Agric. Food Chem., Vol. 60, No.7, 1628–1634, 2012. [9]. H. Oda, A. Kawasaki, and T. Hirata, “Determination of the geographic origin of brown- rice with isotope ratios of 11 B/ 10 B and 87 Sr/ 86Sr”, Anal. Sci., Vol. 17, pp. i1627–i1630, 2001. [10]. P.Martin, M.Madeira, F.Monteiro, R.Bruno de Sousa, A.S.Curvelo-Garcia and S.Catarino, “87Sr/86Sr ratio in vineyard soils from portuguese denominations of origin and its potential from origin authentication”, J. Int. Sci. Vigne Vin, 48, No 1, 21-29, 2014. [11]. C.M.Almeida and M.T.S.D. Vasconcelos, “ICP-MS determination of strontium isotope ratio in wine in order to be used as a fingerprint of its regional origin”, J.Anal. At. Spectrom., 16, 607-611, 2001. [12]. C.Vorster, T.N. van der Walt, P.P. Coetzee, “Ion exchange separation of strontium and rubidium on Dowex 50W-X8, using the complexation properties of EDTA and DCTA”, Anal. Bioanal. Chem. 392, 287-296, 2008. NGUYEN THI KIM DUNG, THAI THI THU THUY 43 [13]. Ţ. Grahek, K. Košutić, and S. Lulić, “Improved method for the separation of radioactive strontium from various samples by mixed solvent anion exchange”, J. Radioanal. Nucl. Chem., vol. 242, No.1, pp. 33–40, 1999. [14]. Ţ. Grahek, I. Eškinja, K. Košutic, S. Lulic, and K. Kvastek, “Isolation of radioactive strontium from natural samples: Separation of strontium from alkaline and alkaline earth elements by means of mixed solvent anion exchange”, Anal. Chim. Acta, vol. 379, no. 1–2, pp. 107– 119, 1999. [15]. V.E. Noshkin and N.S. Mott, “Separation of strontium from large amounts of calcium, with application to radiostrontium analysis”, Talanta, Vol.14, pp. 45 to 51, 1967. [16]. E.P. Horwitz, m.L. Dietz, and D. E. Fisher, “Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using crown ether”, Anal.Chem., 63, 522-525, 1991. [17]. Ţ. Grahek, I. Eškinja, Š. Cerjan, K. Kvastek, S. Lulić, “Separation of Strontium from Calcium by means of Anion Exchanger and Alcoholic solution of Nitric acid”, J. Radioanal. & Nucl. Chem., Vol.182, No2, 401- 410, 1994. [18]. M.V. Zoriy, A. Rashad, C. Pickhardt, H.T. Mohsen, H. Foerstel, A.I. Helal, N.F. Zahran, and J.S. Becker, “Routine method for 87Sr/86Sr isotope ratio measurement in biological and geological samples after matrix separation by ICP-MS”, Atomic Spectroscopy, Vol. 24(6), pp. 195-199, 2003. INSTRUCTIONS FOR AUTHORS GENERAL INFORMATION Nuclear Science and Technology (NST), an international journal of the Vietnam Atomic Energy Society (VAES) and Vietnam Atomic Energy Institute (VINATOM), quarterly publishes articles related to theory and application of nuclear science and technology. All papers and technical notes will be refereed. It is understood that the paper has been neither published nor currently submitted for publication elsewhere. The copyright of all published papers and notes will be transferred in VAES. DETAILED FIELDS NST coves all fields of nuclear science and technology for peaceful utilization of nuclear energy and radiation. Authors should choose one of the following fields at the time they submit their manuscript: 1) Nuclear Physics, 2) Nuclear Data, 3) Reactor Physics, 4) Thermal Hydraulics, 5) Nuclear Safety, 6) Nuclear I&C, 7) Nuclear Fuel and Materials, 8) Radioactive Waste Management, 9) Radiation Protection, 10) Radiation Technology, 11) Nuclear Techniques in Food and Agriculture, 12) Nuclear Medicine and Radiotherapy, 13) Nuclear Techniques in Industries, 14) Environment Radioactivity, 15) Isotope Hydrology, 16) Nuclear Analytical Methods, 17) Health Physics, 18) Fusion and Laser Technology. MANUSCRIPT SUBMISSION Manuscript for publication should be submitted to the Editorial Office in triplicate by postal mail. For electronical submission use nuscitech@vinatom.gov.vn. Submission Address Department of Planning, R&D Management Vietnam Atomic Energy Institute, 59 Ly Thuong Kiet Street, Hanoi, Vietnam E-mail: nuscitech@vinatom.gov.vn. MANUSCRIPT PREPARATION Manuscripts must be written in English with adequate margins and indented paragraph. All manuscript must use SI (metric) units in text, figures, and tables. Manuscripts should in general be organized in the following order: title, names of authors and their complete affiliation including zip code, abstract (not exceeding 200 words), keywords (up to 7), introduction, main body of a paper, acknowledgments, references, appendices, table & figure captions, tables and figures. Unnecessary sections may be omitted. Headings: Use I, II, for major headings and A, B, for secondary headings. Mathematical formulas: All mathematical formulas should be clearly written, with special consideration to distinctive legibility of sub-and superscripts. Equation (at least the principal ones) should be numbered consecutively using Arabic numerals in parentheses in the right hand margin. Tables and Figures: Tables should be numbered with Roman numerals. Figures should be numbered consecutively with Arabic numerals in order of their first appearance and have a complete descriptive title. They should be typed on separate sheets. Tables should no repeat data which are available elsewhere in the paper. Figures should be original ink drawing or computer drawn figures in the original and of high quality, ready for direct reproduction. Figures should be referred to in the text as, for example, Fig. 1., or Fig. 2. . Reference: References should be listed at the end of the text and presented as follows: [1] C. Y. Fu et al., Nuclear Data for Science and Technology, S. M. Qaim (Ed.), p. 587 (1991). [2] C. Kalbach, Z. Phys, A283, 401 (1977). [3] S. Shibata, M. Imamura, T. Miyachi and M. Mutou, “Photonuclear spallation reactions in Cu”, Phys. Rev. C 35, 254 (1987). KHOA HỌC VÀ CÔNG NGHỆ HẠT NHÂN Chịu trách nhiệm xuất bản TRẦN HỮU PHÁT Chịu trách nhiệm nội dung TRẦN HỮU PHÁT TRẦN CHÍ THÀNH Trình bày LÊ THÚY MAI In 200 cuốn, khổ 19x26,5cm tại Công ty TNHH Trần Công Địa chỉ: số 12 ngách 155/176 Đường Trường Chinh, Hà Nội Giấy đăng ký kế hoạch xuất bản số: 770/GP-BTTTT cấp ngày 20 tháng 5 năm 2011 In xong và nộp lưu chiểu Quý I năm 2019 25 000đ
File đính kèm:
 nuclear_science_and_technology_volume_8_number_4_december_20.pdf
nuclear_science_and_technology_volume_8_number_4_december_20.pdf

