Nuclear Science and Technology - Volume 10, Number 1, March 2020
Abstract The isomeric ratio (IR) of isomeric pair 109m,gPd, produced in 110Pd(γ, n)109m,gCd reaction
and 108Pd(n, γ)109m,gPd neutron capture reactions, induced by thermal, epithermal and mixed
thermal-epithermal neutrons have been determined. The off-line activation technique using a
spectroscopic system consisting of a HPGe semiconductor detector with high energy resolution and
a PC based 8192 channel analyzer (CANBERRA) was applied. The investigated samples were
prepared from the 99.99 % purity PdO and irradiated at the electron accelerator Microtron MT-25
of the Joint Institute for Nuclear Research Dubna, Russia. The data analysis and necessary
corrections were made to upgrade the precision of the experimental method. The obtained results
were discussed, compared and combined with those from other authors to point out the role of the
reaction channels in nuclear reactions.
Keywords: Photonuclear reaction - neutron capture reactions - isomeric ratio – isomeric pair
109m,gPd – intake impulse – transfer momentum - reaction channel effect.
I. INTRODUCTION
The product of a nuclear reaction can
exist in the isomeric or ground states. The ratio
of the cross sections or the yields (in the case
of continuous excitation energy spectrum) of
these two states is the so called isomeric ratio
(IR), which furnishes important information on
nuclear level structure, level density, namely
the spin cut-off parameter σ and the level
density parameter a as well as nuclear reaction
mechanism. In fact this ratio is connected to
different nuclear effects as the excitation
energy, momentum transfer, spins of the
isomeric and ground states and their
dependence, nucleon configuration,
intermediate state structure, nuclear channel
effect, the contributions of direct and preequilibrium processes and so on [1 - 16]. N.
Tsoneva et al [3] measured the IRs in (γ, n)
reactions in N = 81 isotone nuclei (137Ba, 139Ce,
141Ba and 143Ba) and showed that in the isotone
nuclei, the IRs depend on the mass numbers.
This is the so called nucleon configuration
effect and was also observed in [3 - 6]. In ref.
[4] we have studied the IRs of (γ, n) nuclear
reactions in Z = 56 isotopes of Ba and the
isomeric pairs 129m,gBa, 131m,gBa and 133Ba were
formed with the same spin ground state 1+/2
and isomeric states of spins 7+/2, 9-/2 and 11-/2,
respectively. The results showed that the IR
decreases with the increase of the isomeric state spin

Trang 1

Trang 2

Trang 3

Trang 4

Trang 5
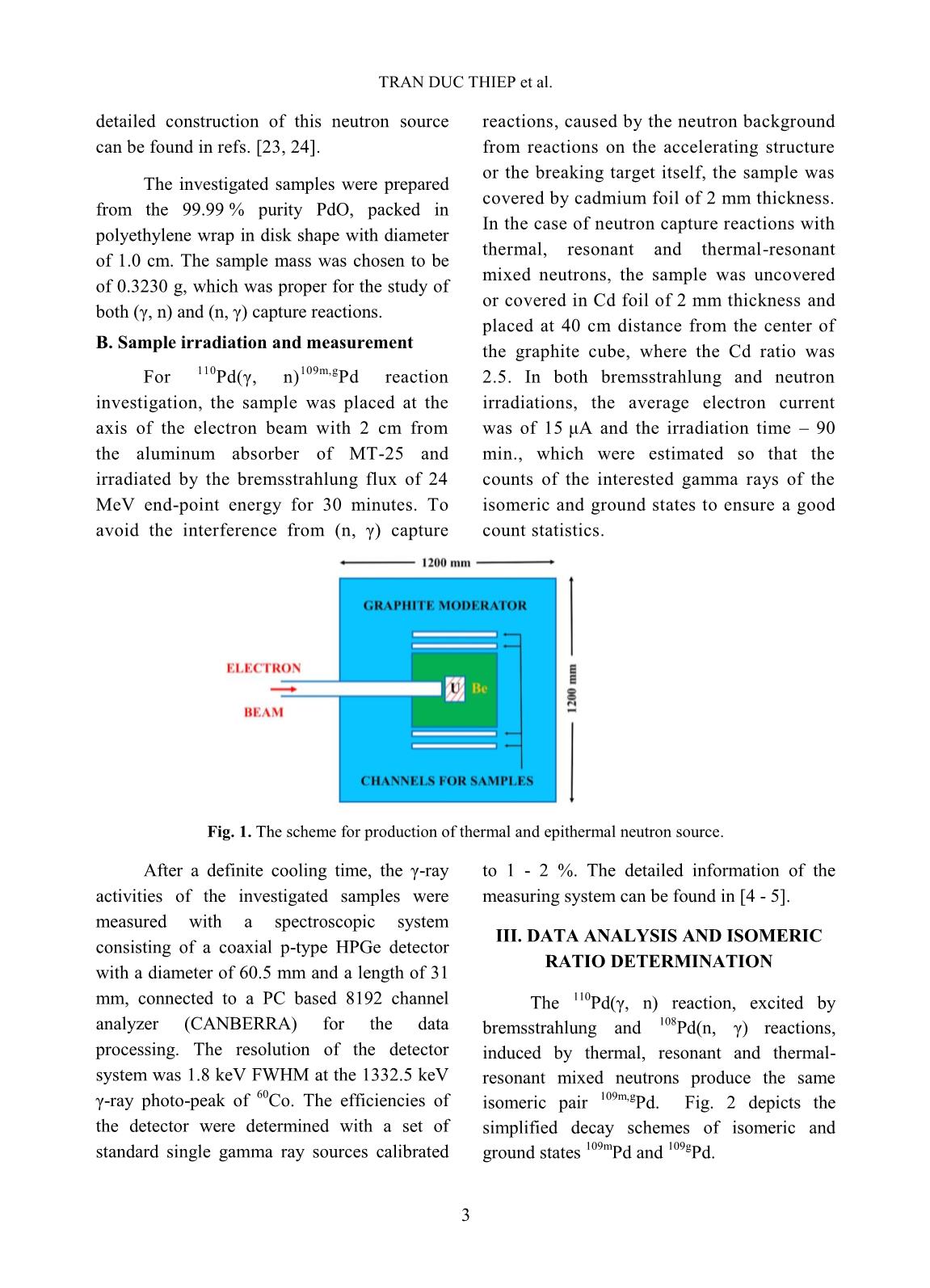
Trang 6
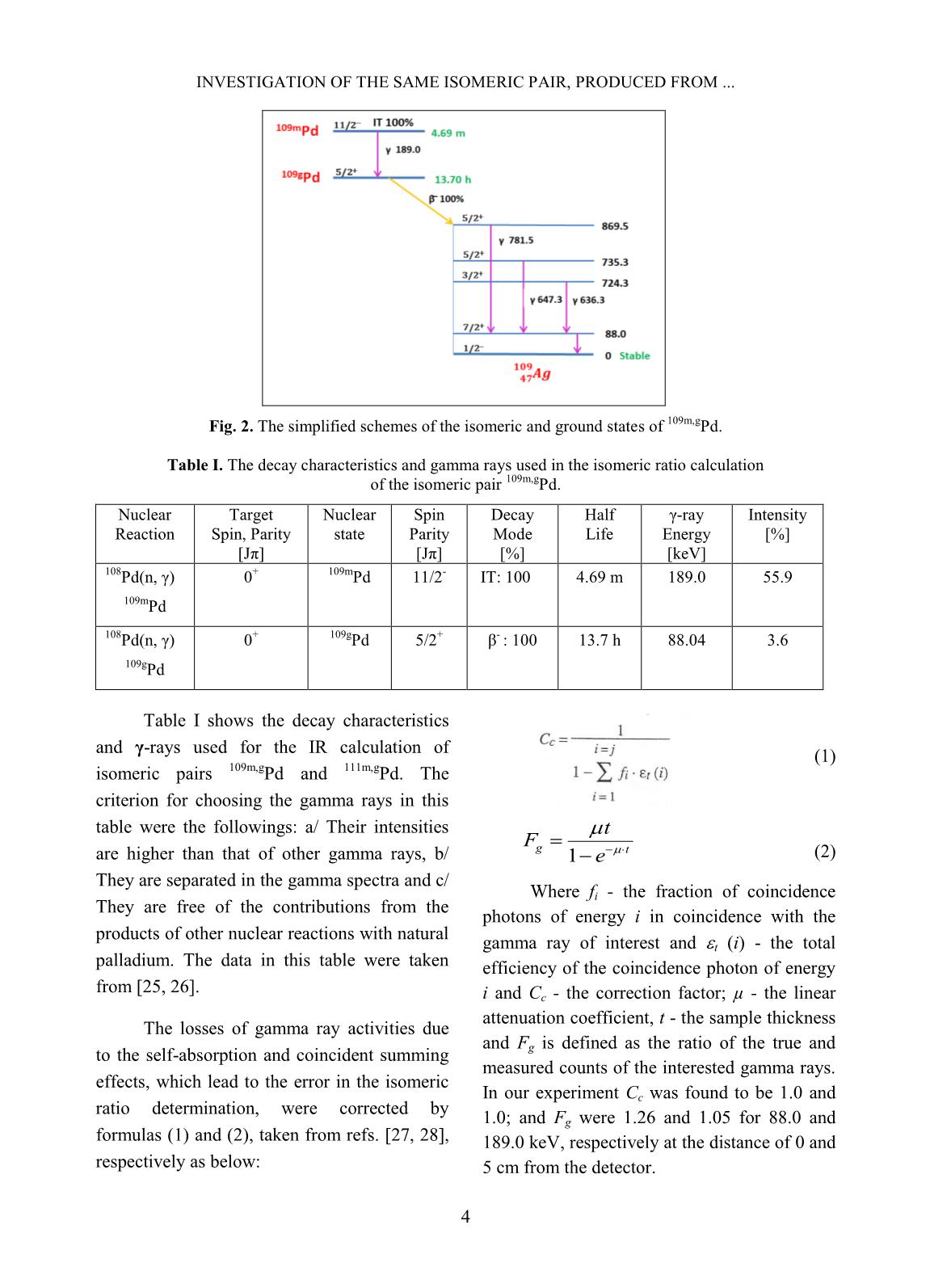
Trang 7
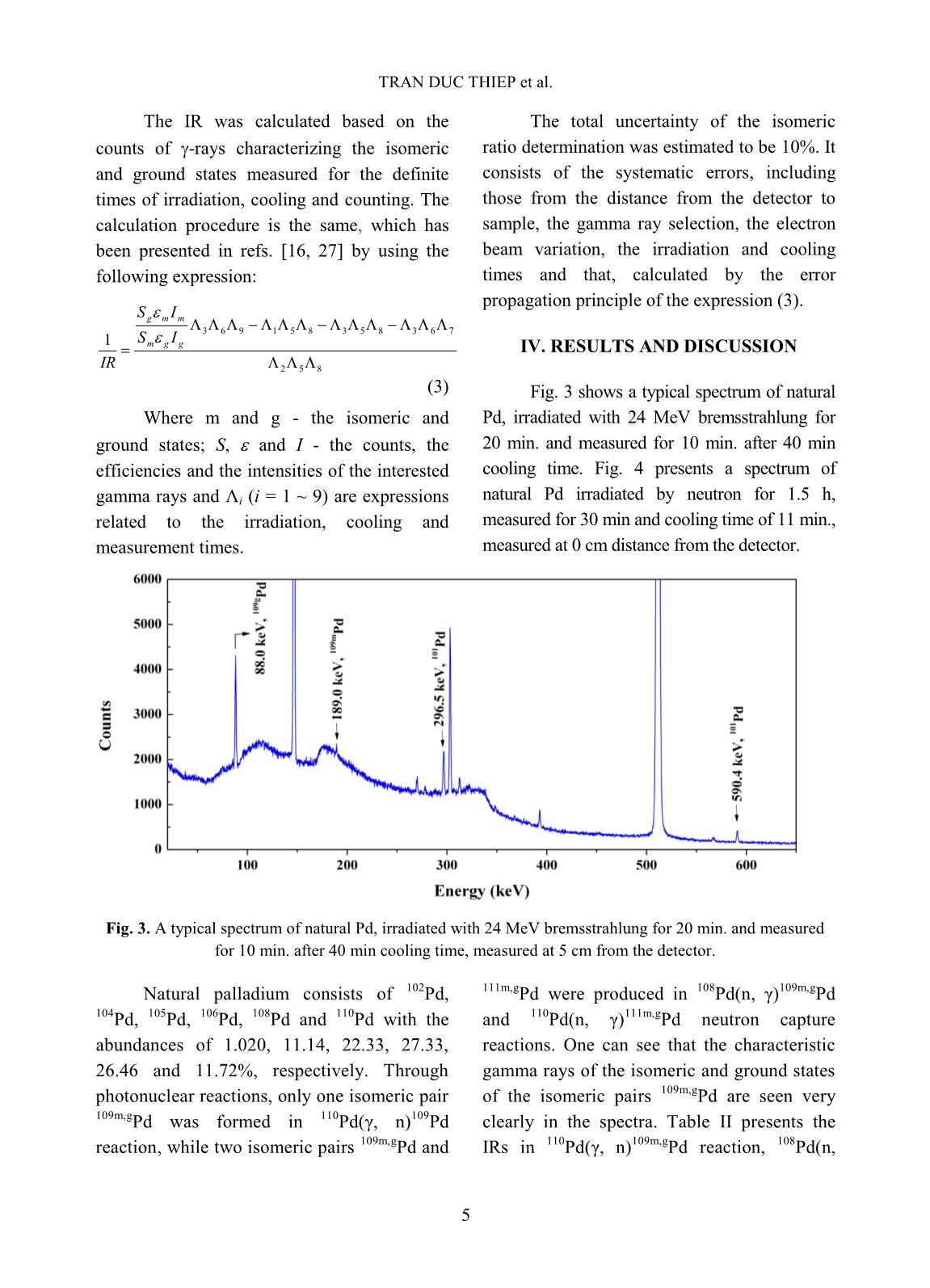
Trang 8
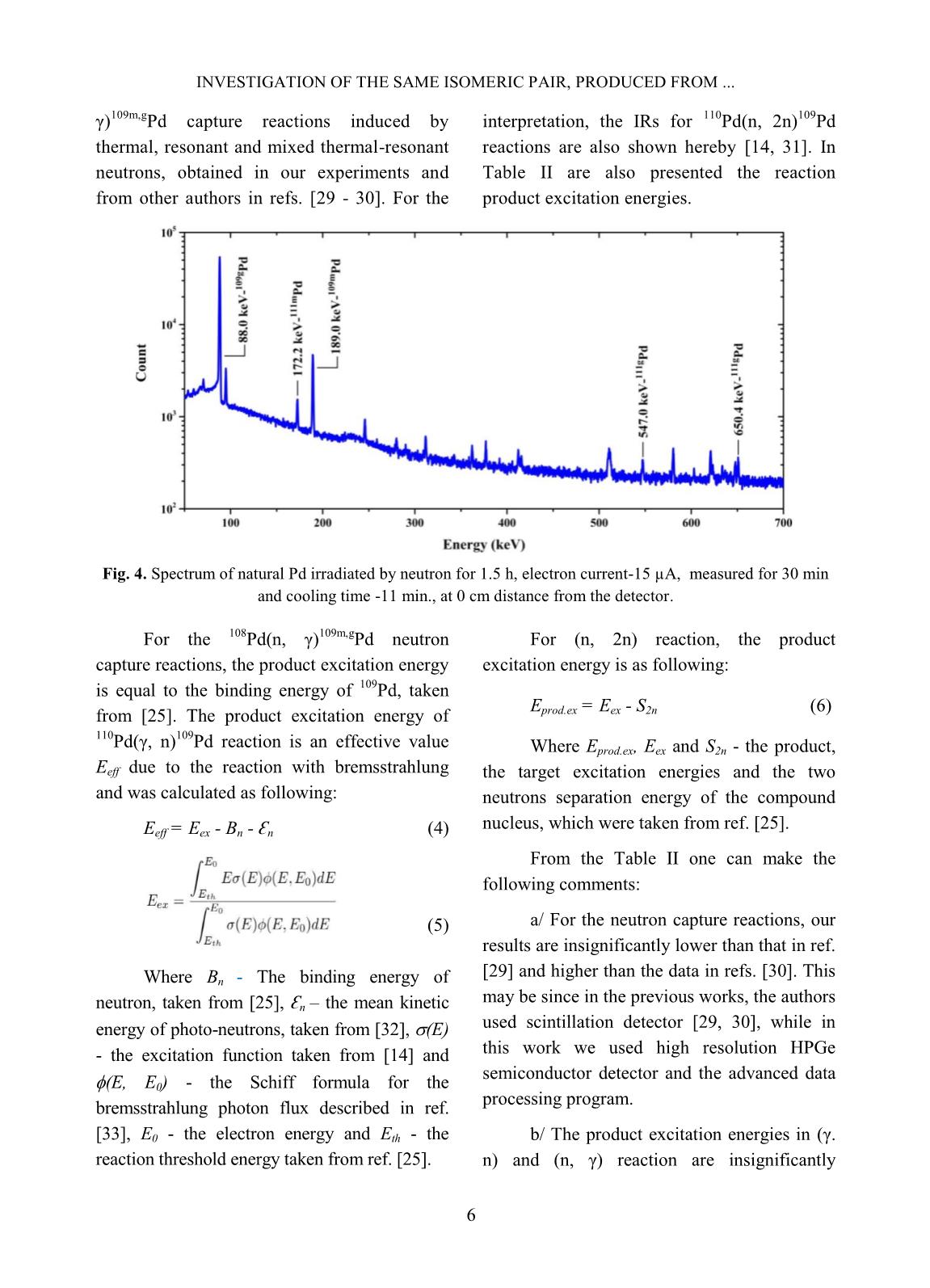
Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Nuclear Science and Technology - Volume 10, Number 1, March 2020

ty The results presented in Fig. 3 indicated that all chitosan- contaning solutions (CTS and CTS-GA solutions) exhibited ATBS •+ radical scavenging ability while GA solution did not. Moreover, the ATBS •+ radical scavenging ability of chitosan solution was higher than CTS-GA solution (A0 sample). This finding was suggested to be due to the obstacle of glucosamine over chitosan on scavenging ATBS •+ radical. Furthermore, the irradiated CTS-GA solutions manifested high ability on scavenging ATBS •+ radical in dependence on the irradiation dose and reaction time, namely the higher irradiation dose and/or reaction time the higher ATBS •+ radical scavenging capacity. The formation of antioxidant compound by heating sugar-amino solution has been reported [23]. This result indicated that MRPs formed upon irradiation of chitosan-glucosamine solution possessed significant antioxidant potential. In addition, the dose-dependent antioxidant activity of irradiation treating chitosan-glucose solution has also been recorded in other study [16]. As the above discussion, during irradiation treatment, the early MRPs was almost saturated at the dose of 25 kGy, while the late MRPs were produced continuously up to 100 kGy, so this results suggested that the formation of antioxidant compounds were mainly taken place at the late stage of Maillard reaction. Fig. 4. The result of agar well diffusion test (GA: glucosamine; A0, A25, A50 and A100 were the A solutions irradiated with the dose of 0, 25, 50, 75 and 100 kGy respectively). LE ANH QUOC et al. 53 Evaluation of antibacterial activity In Fig. 4, the A solutions prepared at different irradiation doses were able to form inhibition zone against E. coli while the GA sample was not. This meant that glucosamine did not exhibit the antibacterial activity in contrast to other A samples. Interestingly, around the well of A0 sample (a CTA-GA solution without irradiation), the presence of inhibition zone indicated that the antibacterial activity of this solution was due to the role of chitosan. The antibacterial ability of samples could be primarily compared through the diameters of their inhibition zones formed on the plate [21], therefore the result indicated that the antibacterial activity decreased obviously in A25, A50, A75 and A100 sample respectively. Fig. 5. Viable bacteria density of the suspension after exposing time (A0, A25, A50 and A100 were the A solutions irradiated with the dose of 0, 25, 50, 75 and 100 kGy respectively). Modification of chitosan via Maillard reaction has been widely studied and it is suggested that MRPs produced from chitosan- sugar model system have been associated with the formation of compounds with high antibacterial [16, 22, 24]. However, there is no information of the influence of irradiation dose on the information of these compounds. Thus, in this study the antibacterial activities of MPRs prepared with different dose were examined and further compared with chitosan. Namely, after exposing time, the bacterial cell density of the suspensions at pH 5 and 7 was determined and described in Fig. 5. The result revealed that the antibacterial activity of A0 sample was affected deeply by the pH value, namely been high at pH 5 and low at pH 7. As above discussion, the antibacterial activity of A0 sample was mainly contributed by chitosan, which was precipitated and reduced its bioactivity at neutral or alkaline solution, hence the antibacterial activity of A0 sample at pH 5 was greater than at pH 7. This finding is totally in concurrence with other studies where the pH-dependent antibacterial activity of chitosan has been reported [17, 25]. In addition, the obtained results also indicated that all testing samples had the lower bacterial cell density in comparison with the control, this meant that these samples exhibited an effective antibacterial activity against E. coli at both pH 5 and 7. The lower viable bacteria density represented the stronger antibacterial activity. Therefore at pH 5 and 7, the antibacterial activity of irradiated samples decreased along with the increasing dose and A25 was the most antibacterial sample. This record completely matched with the results of agar well diffusion MAILLARD REACTION PRODUCTS OF CHITOSAN AND GLUCOSAMINE 54 test above. Furthermore, the higher antibacterial activity of chitosan-glucosamine derivatives prepared by heat-induced Maillard reaction than acid-soluble chitosan was also recorded in the study of Chung et al. (2005). In addition, because during irradiation treatment, the early MRPs were created prior to the formation of late MRPs so the results above suggested that the antibacterial activities of irradiated solutions were probably due to the role of early MRPs. Interestingly, the antibacterial activities of MRPs in irradiated solutions were maintained at high level at both pH 5 and 7. Hence, this result is one of the most demonstrations of Maillard reaction effectiveness in chitosan modification strategies. III. CONCLUSIONS This study demonstrated that the Maillard reaction can be easily occurred in the CTS-GA admixture solution by gamma irradiation. Moreover, the concentration of glucosamine suitable for Maillard reaction is much lower than the concentration of chitosan in the CTS-GA admixture solution, namely about 0.18% GA for 1% CTS (w/w), and as- prepared CTS-GA MRPs exhibited high antioxidant activity and strong antibacterial activity at pH 5 and 7. These findings indicated that CTS-GA MRPs can be used as potential natural products replacing for synthetic additives in food or cosmetic. Further studies are necessary to elucidate mechanism of compounds formed during irradiation treatment and their application in practice. REFERENCES [1]. Lucera, A., Costa, C., Conte, A., Nobil, M.A.D., "Food applications of natural antimicrobial compounds", Frontiers in Microbiology, 3: 27, 2012. [2]. Harish Prashanth, K.V., Tharanathan, R.N., “Chitin/chitosan: modifications and their unlimited application potential – an overview”, Trends in Food Science & Technology, 18, pp. 117-13, 2007. [3]. Rudrapatnam, N.T., Farooqahmed, S.K., "Chitin - The undisputed biomolecule of great potential", Critical Review in Food Science ans Nutrion, 43, 61-87, 2003. [4]. Rao, M.S., Chander, R., Sharma, A., "Development of shelfstable intermediate- moisture meat products using active edible coating and irradiation", Journal of Food Science, 70, 325-331, 2005. [5]. Roller, S., Covill, N., "The antimicrobial properties of chitosan in mayonnaise and mayonnaise-based shrimp salads", Journal of Food Protection, 63, 202–209, 2000. [6]. Weiner, M.L., "An overview of the regulatory status and of the safety of chitin and chitosan as food and pharmaceutical ingredients", Advances in Chitin and Chitosan - Edited by C. J. Brine, P. A. Sandford and J. P. Zikakis. Elsevier Science Publishers, London, 663-670, 1992. [7]. Korea Food and Drug Administration [KFDA]. Food additives code. Seoul, Korea: KFDA, 1995. [8]. Sagoo, S., Board, R., Roller, S., "Chitosan inhibits growth of spoilage microorganisms in chilled pork products", Food Microbiology, 19, 175–182, 2002. [9]. Chien, P.J., Sheu, F., Lin, H.R., "Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life", Food Chemistry, 100(3), 1160- 1164, 2007. [10]. Badawy, M.E.I., Rabea, E.I., "Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit", Postharvest Biology and Technology, 51(1): 110-117, 2009. [11]. Tsai, G.J., SU, W.H., Chen, H.C., Pan, C.L., "Antimicrobial activity of shrimp chitin and LE ANH QUOC et al. 55 chitosan from different treatments and applications of fish preservation", Fisheries Science, 68(1): 170-177, 2002. [12]. Darmadji, P., Izumimoto, M., “Effect of chitosan in meat preservation”, Meat Science, 38(2), pp. 243-254, 2004. [13]. Chang, H.L., Chen, Y.C., Tan, F.J., "Antioxidative properties of a chitosan-glucose Maillard reaction product and its effect on pork qualities during refrigerated storage", Food Chemistry, 124, 589–595, 2011. [14]. Kanatt, S.R., Chander, R., Sharma, A., “Chitosan glucose complex – A novel food preservative”, Food Chemistry, 106, pp. 521-528, 2008. [15]. Maillard, M.N., Billaud, C., Chow, Y.N., Ordonaud, C., Nicolasb, J., "Free radical scavenging, inhibition of polyphenoloxidase activity and copper chelating properties of model Maillard systems", LWT - Food Science and Technology, 40, 1434–1444, 2007. [16]. Rao, M.S., Chawla, S.P., Chander, R., Sharma, A., "Antioxidant potential of Maillard reaction products formed by irradiation of chitosan- glucose solution", Carbohydrate Polymers, 83, pp. 714-719, 2011. [17]. Chung, Y.C., Kuo, C.L., Chen, C.C., “Preparation and important functional properties of water-soluble chitosan produced through Maillard reaction”, Bioresource Technology, 96, pp. 1473-1482, 2005. [18]. Chawla, S.P., Chander, R., Sharma, A., "Antioxidant properties of Maillard reaction products by gamma-irradiation of whey protein", Food Chemistry, 116, pp. 122-128, 2009. [19]. Zhai, X., Zhang, C., Zhao, G., Stoll, S., Ren, F., Leng, X., "Antioxidant capacities of the selenium nanoparticles stabilized by chitosan", Journal of Nanobiotechnology, 15 (4). [20]. Chen, W., Li, Y., Yang, S., Yue, L., Jiang, Q., Xia, W., "Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan- stabilized selenium nanoparticles", Carbohydrate Polymers, 132, 574-581, 2015. [21]. Balouiri, M., Sadiki, M., Ibnsouda, S.K., "Methods for in vitro evaluating antimicrobial activity: A review", Journal of Pharmaceutical Analysis, 6, pp. 71-79, 2016. [22]. Chawla, S.P., Chander, R., Sharma, A., "Antioxidant formation by γ-irradiation of glucose-amino acid model system", Food Chemistry, 103, pp. 1297-1340, 2007. [23]. Lingnert, H., Eriksson, C.E., "Antioxidative Maillard reaction products. II. Products from sugars and peptides or protein hydrolysates", Journal of Food Processing and Preservation, 4, 173–181, 1980. [24]. Mahae, N., Chalat, C., Muhamud, P., "Antioxidant and antimicrobial properties of chitosan-sugar complex", International Food Research Journal, 18(4), 1543-1551, 2011. [25]. Kulikov, S., Tikhonov, V., Blagodatskikh, I., Bezrodnykh, E., Lopatin, S., Khairullin, R., Philippova, Y., Abramchuk, S., "Molecular weight and pH aspects of the efficacy of oligochitosan against methicillin-resistant Staphylococcus aureus (MRSA)", Carbohydrate Polymers, 87(1), 545-550, 2012. INSTRUCTIONS FOR AUTHORS GENERAL INFORMATION Nuclear Science and Technology (NST), an international journal of the Vietnam Atomic Energy Society (VAES) and Vietnam Atomic Energy Institute (VINATOM), quarterly publishes articles related to theory and application of nuclear science and technology. All papers and technical notes will be refereed. It is understood that the paper has been neither published nor currently submitted for publication elsewhere. The copyright of all published papers and notes will be transferred in VAES. DETAILED FIELDS NST coves all fields of nuclear science and technology for peaceful utilization of nuclear energy and radiation. Authors should choose one of the following fields at the time they submit their manuscript: 1) Nuclear Physics, 2) Nuclear Data, 3) Reactor Physics, 4) Thermal Hydraulics, 5) Nuclear Safety, 6) Nuclear I&C, 7) Nuclear Fuel and Materials, 8) Radioactive Waste Management, 9) Radiation Protection, 10) Radiation Technology, 11) Nuclear Techniques in Food and Agriculture, 12) Nuclear Medicine and Radiotherapy, 13) Nuclear Techniques in Industries, 14) Environment Radioactivity, 15) Isotope Hydrology, 16) Nuclear Analytical Methods, 17) Health Physics, 18) Fusion and Laser Technology. MANUSCRIPT SUBMISSION Manuscript for publication should be submitted to the Editorial Office in triplicate by postal mail. For electronical submission use nuscitech@vinatom.gov.vn. Submission Address Department of Planning, R&D Management Vietnam Atomic Energy Institute, 59 Ly Thuong Kiet Street, Hanoi, Vietnam E-mail: nuscitech@vinatom.gov.vn. MANUSCRIPT PREPARATION Manuscripts must be written in English with adequate margins and indented paragraph. All manuscript must use SI (metric) units in text, figures, and tables. Manuscripts should in general be organized in the following order: title, names of authors and their complete affiliation including zip code, abstract (not exceeding 200 words), keywords (up to 7), introduction, main body of a paper, acknowledgments, references, appendices, table & figure captions, tables and figures. Unnecessary sections may be omitted. Headings: Use I, II, for major headings and A, B, for secondary headings. Mathematical formulas: All mathematical formulas should be clearly written, with special consideration to distinctive legibility of sub-and superscripts. Equation (at least the principal ones) should be numbered consecutively using Arabic numerals in parentheses in the right hand margin. Tables and Figures: Tables should be numbered with Roman numerals. Figures should be numbered consecutively with Arabic numerals in order of their first appearance and have a complete descriptive title. They should be typed on separate sheets. Tables should no repeat data which are available elsewhere in the paper. Figures should be original ink drawing or computer drawn figures in the original and of high quality, ready for direct reproduction. Figures should be referred to in the text as, for example, Fig. 1., or Fig. 2. . Reference: References should be listed at the end of the text and presented as follows: [1] C. Y. Fu et al., Nuclear Data for Science and Technology, S. M. Qaim (Ed.), p. 587 (1991). [2] C. Kalbach, Z. Phys, A283, 401 (1977). [3] S. Shibata, M. Imamura, T. Miyachi and M. Mutou, “Photonuclear spallation reactions in Cu”, Phys. Rev. C 35, 254 (1987). KHOA HỌC VÀ CÔNG NGHỆ HẠT NHÂN Chịu trách nhiệm xuất bản TRẦN HỮU PHÁT Chịu trách nhiệm nội dung TRẦN HỮU PHÁT TRẦN CHÍ THÀNH Trình bày LÊ THÚY MAI In 200 cuốn, khổ 19x26,5cm tại Công ty TNHH Trần Công Địa chỉ: số 12 ngách 155/176 Đường Trường Chinh, Hà Nội Giấy đăng ký kế hoạch xuất bản số: 770/GP-BTTTT cấp ngày 20 tháng 5 năm 2011 In xong và nộp lưu chiểu Quý I năm 2020 25 000đ
File đính kèm:
 nuclear_science_and_technology_volume_10_number_1_march_2020.pdf
nuclear_science_and_technology_volume_10_number_1_march_2020.pdf

