Determination of recovery efficiency of 137Cs in seawaterusing co-precipitation method
Recovery efficiency of 137Cs in seawater samples with different volumes of 40, 50, 60, 80
and 100 liters using co-precipitation method by ferrocyanide compounds has been determined. 134Cs
nuclide was used as a tracer to determine recovery efficiency. The results showed that the recovery
efficiency of 134Cs ranged from 92.62% to 99.26% with mean value of (95.22 ± 2.61)% for different
sample volumes. Average recovery efficiency for samples with a volume of 50 liters was (95.70 ±
2.50)% and uncertainty when determining 137Cs in seawater samples were still less than 20%.
Therefore, reducing the volume of sample to 50 liters still ensures reliability when determining 137Cs
in seawater samples by co-precipitation method, thereby reducing the chemical and time when analyze
a large number of samples.

Trang 1

Trang 2
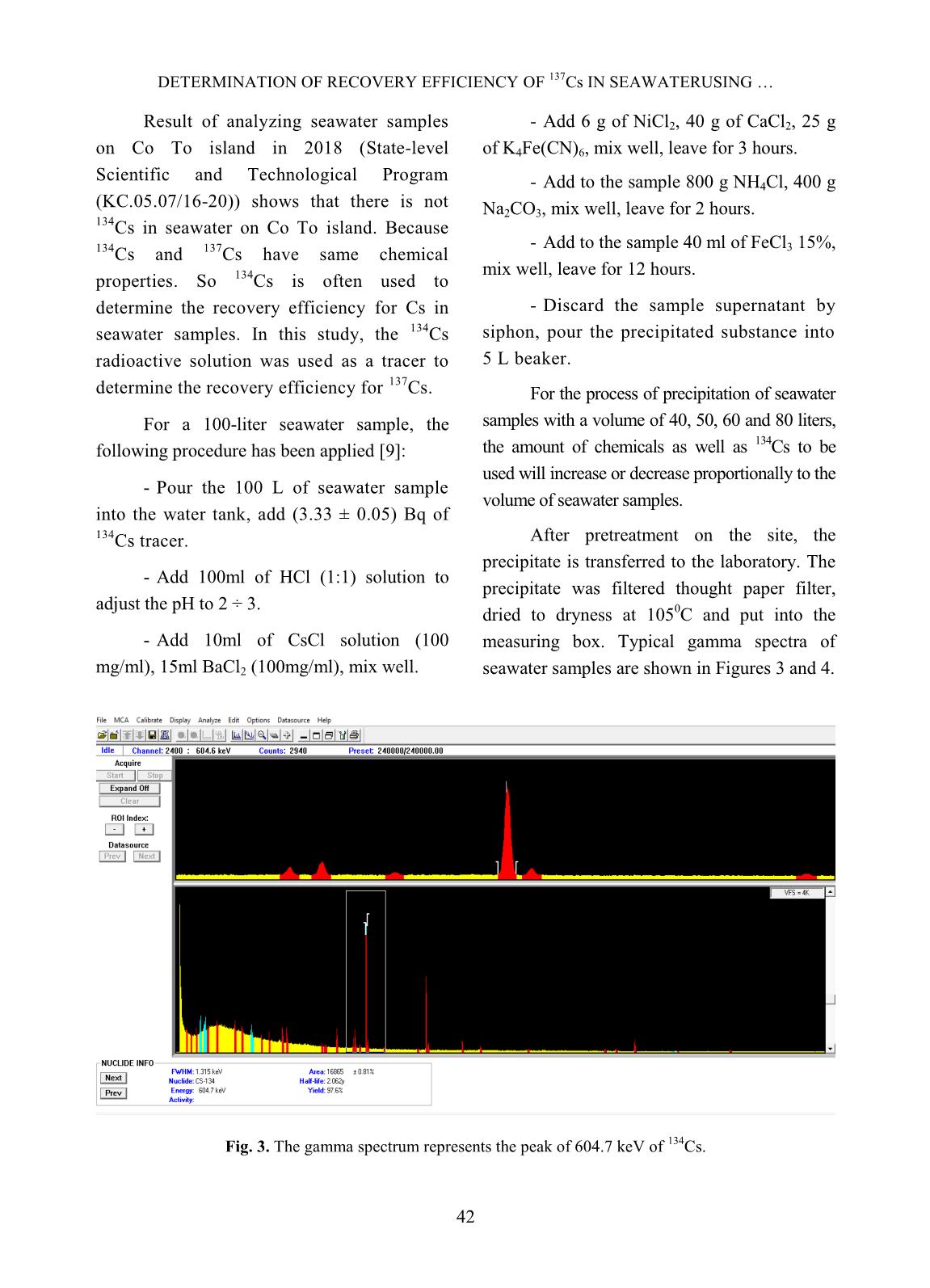
Trang 3
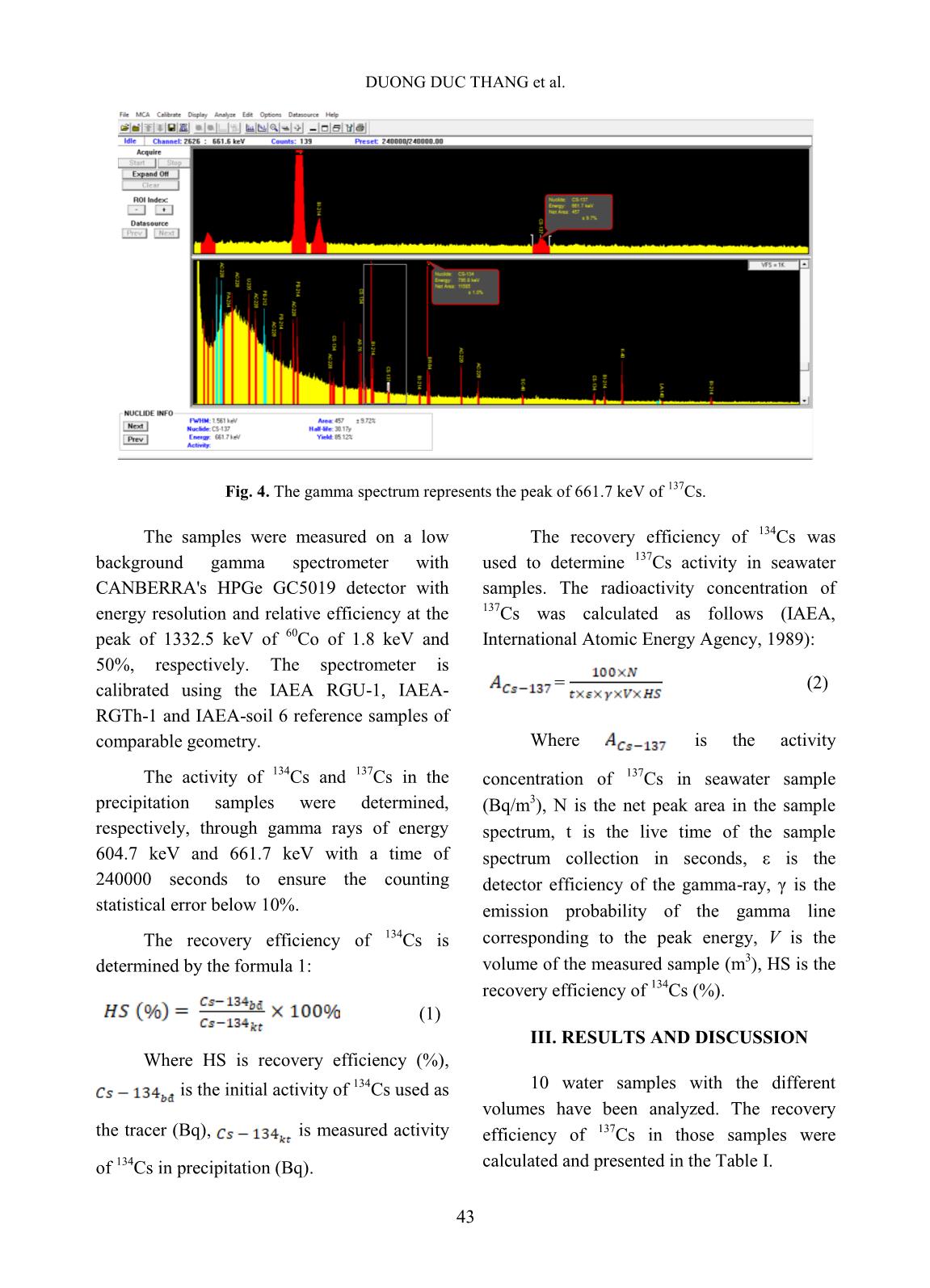
Trang 4
Trang 5
Trang 6

Trang 7
Tóm tắt nội dung tài liệu: Determination of recovery efficiency of 137Cs in seawaterusing co-precipitation method

ergy Institute Determination of recovery efficiency of 137 Cs in seawaterusing co-precipitation method Duong Duc Thang*, Vuong Thu Bac, Bui Dac Dung, Duong Van Thang, Doan Thuy Hau, Le Dinh Cuong, Nguyen Van Khanh, Nguyen Thi Thu Ha, Cao Duc Viet, Nguyen Huyen Trang, Nguyen Thi Oanh, Le Thị Hoa Institute for Nuclear Science and Technology, 179 Hoang Quoc Viet – Cau Giay – Hanoi * Email: ducthangb2k52@gmail.com, thangdd@vinatom.gov.vn (Received 16 December 2019, accepted 04 March 2020) Abstract: Recovery efficiency of 137 Cs in seawater samples with different volumes of 40, 50, 60, 80 and 100 liters using co-precipitation method by ferrocyanide compounds has been determined. 134 Cs nuclide was used as a tracer to determine recovery efficiency. The results showed that the recovery efficiency of 134 Cs ranged from 92.62% to 99.26% with mean value of (95.22 ± 2.61)% for different sample volumes. Average recovery efficiency for samples with a volume of 50 liters was (95.70 ± 2.50)% and uncertainty when determining 137 Cs in seawater samples were still less than 20%. Therefore, reducing the volume of sample to 50 liters still ensures reliability when determining 137 Cs in seawater samples by co-precipitation method, thereby reducing the chemical and time when analyze a large number of samples. Keywords: Recovery efficiency of 137 Cs, co-precipitation method, seawater, HPGe. I. INTRODUCTION Huge amounts of anthropogenic radionuclides have been introduced into marine environment as global fallout from large-scale atmospheric nuclear-weapon testing, discharge from nuclear facilities and ocean dumping of nuclear wastes [1]. Their radiological and ecological effects are still of world concern [2]. 137 Cs is one of the most important anthropogenic radionuclides in the field of environmental radioactivity monitoring because of its long physical half-life of 30.02 years. It is a major fission product (fission yield is about 6–7%) from both plutonium and uranium [1]. 137 Cs in the ocean has been mainly derived from global fallout [1, 2, 3, 4, 5], together with close-in fallout from the Pacific Proving Ground nuclear explosions [3, 4], discharge of radioactive wastes from nuclear facilities and others [6, 7, 8]. The determination of activity concentration of radionuclides in seawater samples is a complicated issue, because their activity in seawater samples is very low, a large number of samples are required (about 200 - 400 liters) [9]. Currently, there are various processes in the world for preliminary enrichment of seawater samples on the field before laboratory analysis. A number of previous studies have successfully reduced seawater sample volume to 20 liters [10, 11] or 10 liters [12] to reduce chemical and sample processing time while still ensuring accuracy. This paper presents the survey results on the recovery efficiency of 137 C in seawater samples by different volumes, determining the activity of 137 C and uncertainty by co- precipitation method in the field then analysis in the laboratory of Center for Environmental Radiation Monitoring and DUONG DUC THANG et al. 41 Impact Assessment (CERMIA), Institute for Nuclear Science and Technology (INST). Based on the results obtained, it is recommended to analyze 137 C in seawater samples with optimal volumes. II. MATERIALS AND METHODS Sampling and sample processing The activity concentration of 137 Cs currently in the East Sea is quite small. It ranges from (1.16 ± 0.06) Bq/m 3 to (1.62 ± 0.15) Bq/m 3 [9], therefore, to determine 137 Cs, the enrichment method must be applied prior to analysis in the laboratory. Seawater samples were taken on Co To island, Co To District, Quang Ninh province, the sampling locations are shown in Figure 1. Samples were taken at the time of high tide to avoid the influence from the mainland. In this study, we took samples of surface seawater with different volumes of 40, 50, 60, 80 and 100 liters, pretreated by co-precipitation method in the field (Figure 2) according to the procedure presented as shown below. Fig. 1. Location of seawater sampling on Co To island. Fig. 2. Sample treatment. DETERMINATION OF RECOVERY EFFICIENCY OF 137 Cs IN SEAWATERUSING 42 Result of analyzing seawater samples on Co To island in 2018 (State-level Scientific and Technological Program (KC.05.07/16-20)) shows that there is not 134 Cs in seawater on Co To island. Because 134 Cs and 137 Cs have same chemical properties. So 134 Cs is often used to determine the recovery efficiency for Cs in seawater samples. In this study, the 134 Cs radioactive solution was used as a tracer to determine the recovery efficiency for 137 Cs. For a 100-liter seawater sample, the following procedure has been applied [9]: - Pour the 100 L of seawater sample into the water tank, add (3.33 ± 0.05) Bq of 134 Cs tracer. - Add 100ml of HCl (1:1) solution to adjust the pH to 2 ÷ 3. - Add 10ml of CsCl solution (100 mg/ml), 15ml BaCl2 (100mg/ml), mix well. - Add 6 g of NiCl2, 40 g of CaCl2, 25 g of K4Fe(CN)6, mix well, leave for 3 hours. - Add to the sample 800 g NH4Cl, 400 g Na2CO3, mix well, leave for 2 hours. - Add to the sample 40 ml of FeCl3 15%, mix well, leave for 12 hours. - Discard the sample supernatant by siphon, pour the precipitated substance into 5 L beaker. For the process of precipitation of seawater samples with a volume of 40, 50, 60 and 80 liters, the amount of chemicals as well as 134 Cs to be used will increase or decrease proportionally to the volume of seawater samples. After pretreatment on the site, the precipitate is transferred to the laboratory. The precipitate was filtered thought paper filter, dried to dryness at 105 0 C and put into the measuring box. Typical gamma spectra of seawater samples are shown in Figures 3 and 4. Fig. 3. The gamma spectrum represents the peak of 604.7 keV of 134 Cs. DUONG DUC THANG et al. 43 Fig. 4. The gamma spectrum represents the peak of 661.7 keV of 137 Cs. The samples were measured on a low background gamma spectrometer with CANBERRA's HPGe GC5019 detector with energy resolution and relative efficiency at the peak of 1332.5 keV of 60 Co of 1.8 keV and 50%, respectively. The spectrometer is calibrated using the IAEA RGU-1, IAEA- RGTh-1 and IAEA-soil 6 reference samples of comparable geometry. The activity of 134 Cs and 137 Cs in the precipitation samples were determined, respectively, through gamma rays of energy 604.7 keV and 661.7 keV with a time of 240000 seconds to ensure the counting statistical error below 10%. The recovery efficiency of 134 Cs is determined by the formula 1: (1) Where HS is recovery efficiency (%), is the initial activity of 134 Cs used as the tracer (Bq), is measured activity of 134 Cs in precipitation (Bq). The recovery efficiency of 134 Cs was used to determine 137 Cs activity in seawater samples. The radioactivity concentration of 137 Cs was calculated as follows (IAEA, International Atomic Energy Agency, 1989): = (2) Where is the activity concentration of 137 Cs in seawater sample (Bq/m 3 ), N is the net peak area in the sample spectrum, t is the live time of the sample spectrum collection in seconds, ε is the detector efficiency of the gamma-ray, γ is the emission probability of the gamma line corresponding to the peak energy, V is the volume of the measured sample (m 3 ), HS is the recovery efficiency of 134 Cs (%). III. RESULTS AND DISCUSSION 10 water samples with the different volumes have been analyzed. The recovery efficiency of 137 Cs in those samples were calculated and presented in the Table I. DETERMINATION OF RECOVERY EFFICIENCY OF 137 Cs IN SEAWATERUSING 44 Table I. Recovery efficiency of 137 Cs in seawater samples by different volumes. Sample Volume (L) Recovery efficiency (%) M1 40 92.62 ± 2.29 M2 40 99.26 ± 2.47 M3 50 98.50 ± 2.24 M4 50 94.54 ± 2.17 M5 50 93.15 ± 2.27 M6 50 93.41 ± 2.25 M7 50 98.92 ± 2.26 M8 60 93.93 ± 2.03 M9 80 93.39 ± 1.93 M10 100 94.48 ± 3.25 Table I shows that the recovery efficiency varies from (92.62 to 98.92)% with an average of (95.22 ± 2.61)%. In which, the recovery efficiency of 137 Cs in different volumes of 40, 50, 60, 80 and 100 liters are (95.94 ± 3.32)%, (95.70 ± 2.50)%, (93.93 ± 2.03)%, (93.39 ± 1.93 )%, (94.48 ± 3.32)%, respectively. The recovery efficiency of 137 Cs in different volume of seawater samples tends to decrease when the volume increases. The average recovery efficiency of the sample volumes of 40 and 50 liters are quite similar, but for our method, the volume of 50 liters is optimal. Because the precipitates obtained from 40-liter samples are approximately the same as the amount to be measured, while the precipitation volume of 50-liter samples is always greater than the amount to be measured. This makes the recovery efficiency of the sample volume of 50 liters stable and has less uncertainty. Fig. 5. The recovery efficiency of 137 Cs versus sample volume. DUONG DUC THANG et al. 45 Figure 5 and Table I shows that the recovery efficiency of 137 Cs in seawater by different sample volumes is quite similar and greater than 90%. The recovery efficiency of the smaller volume seawater samples are greater a bit than those of the larger volume samples. Activity concentrations of 137 Cs in different seawater samples with different volumes calculated by formula 2 are shown in Table II. Table II shows that the activity concentration of 137 Cs are in the range of (1.12 ± 0.23) Bq/m 3 to (1.69 ± 0.21) Bq/m 3 with an average of (1.42 ± 0.22) Bq/m 3 , with uncertainty less than 20%. The activity concentration of 137 Cs in different seawater volumes of this study is similar to previous publication [9]. Table III presents a comparison of the recovery efficiency of 137 Cs in seawater in this study with some results published before. Table II. Activity concentration of 137 Cs in seawater samples. Sample Volume, L Activity concentration 137 Cs (Bq/m 3 ) M1 40 1.12 ± 0.23 M2 40 1.19 ± 0.19 M3 50 1.68 ± 0.17 M4 50 1.42 ± 0.28 M5 50 1.46 ± 0.20 M6 50 1.69 ± 0.21 M7 50 1.14 ± 0.23 M8 60 1.56 ± 0.23 M9 80 1.61 ± 0.21 M10 100 1.29 ± 0.19 Table III. The comparison of the recovery efficiency. Volume, L Recovery efficiency (%) 10 93 ± 3 K. Hirose [10] 20 99 Nakano [11] 20 90 ÷ 95 J. Kamenik [12] 40 92.62 ÷ 99.26 This work 50 93.15 ÷ 98.92 This work 60 93.93 ± 2.03 This work 80 93.39 ± 1.93 This work 100 94.48 ± 3.25 This work From Table III, we find that the recovery efficiency of 137 Cs in seawater in this study is quite good and as similar as results of other papers. IV. CONCLUSIONS In this study, we achieved the initial goal of determining the recovery efficiency of 137 Cs DETERMINATION OF RECOVERY EFFICIENCY OF 137 Cs IN SEAWATERUSING 46 in seawater with different volumes from 40, 50, 60, 80 and 100 liters. The recovery efficiency of 137 Cs in seawater samples varied from 92.62% to 99.26% with an average of (95.22 ± 2.61)% and relatively good repeatability. The average recovery efficiency for samples with a volume of 50 liters is (95.70 ± 2.50)% and the uncertainty when determining the activity concentration of 137 Cs is still less than 20%. Therefore, it is possible to reduce the sample volume to 50 liters while maintaining reliability when determining the activity concentration of 137 Cs in seawater samples by the co-precipitation method, thereby reducing chemicals and time when analyzing many samples at the same time. ACKNOWLEDGMENTS The authors would like to thank Vietnam Atomic Energy Institute (VINATOM) under grant CS/19/04-05 and the State-level Scientific and Technological Program (KC.05.07/16-20) for funding this study. REFERENCE [1]. UNSCEAR. “Sources and Effects of Ionizing Radiation. United Nations”, New York, 2000. [2]. M.Aoyama∗, K.Hirose. “Radiometric determination of anthropogenic radionuclides in seawater”. Radioactivity in the environment, volume 11, 2008. [3]. Bowen, V.T., Noshkin, V.E., Livingston, H.D., Volchok, H.L. “Fallout radionuclides in the Pacific Ocean: vertical and horizontal distributions, largely from GEOSECS stations”. Earth Planet. Sci. Lett. 49, 411–434, 1980. [4]. K. Hirose, Amano, H., Baxter, M.S., Chaykovskaya, E., Chumichev, V.B., Hong, G.H., Isogai, K., Kim, C.K., Kim, S.H., Miyao, T., Morimoto, T., Nikitin, A., Oda, K., Pettersson, H.B.L., Povinec, P.P., Seto, Y., Tkalin, A., Togawa, O., Veletova, N.K. “Anthropogenic radionuclides in seawater in the East Sea/Japan Sea: Results of the first- stage Japanese–Korean–Russian expedition”. J. Environ. Radioact. 43, 1–13, 1999. [5]. Reiter, E.R. Atmospheric Transport Processes. “Part 4: Radioactive Tracers. Technical Information Center”, U.S. Department of Energy, Washington, DC, pp. 1–605, 1978. [6]. Livingston, H.D., Povinec, P.P. “A millennium perspective on the contribution of global fallout radionuclides to ocean science”. Health Phys. 82 (5), 656–668, 2002. [7]. Pentreath, R.J. “Sources of artificial radionuclides in the marine environment”. Radionuclides: A Tool for Oceanography. Elsevier, London, pp. 12–34, 1988. [8]. Sugiura, Y., Saruhashi, K., Miyake, Y.. “Evaluation on the disposal of radioactive wastes into the North Pacific”. Pap. Meteorol. Geophys. 27, 81–87, 1975. [9]. N. Q. Long, L. D. Cuong, T. V. Giap, D. V. Thang, D. D Thang, N. V. Khanh, D. X. Anh. “The dispersion of artificial radionuclides in seawater from the Fukushima nuclear accident to the Eastsea, Vietnam”. The 12th regional conference on nuclear science and technology, 2017. [10]. K. Hirose, M. Aoyama, Y. Igarashi, K. Komura. “Improvement of 137Cs analysis in small volume seawater samples using the Ogoya underground facility”. Journal of Radioanalytical and Nuclear Chemistry, Vol. 276, No.3, 795–798, 2008. [11]. Masanao Nakano, Yuji Kokubun, Takeshi Sasaki, Minoru Takeishi. “Analytical Method of Gamma Emitters in Seawater Using Copercipitation with Nickel Hexacyanoferrate (II) and Iron (III) Hydroxide”. Radioisotopes, 58, 61-69, 2009. [12]. J. Kameník, H. Dulaiova, K.O. Buesseler, S. M. Pike. “Cesium-134 and 137 activities in the central North Pacific Ocean after the Fukushima Dai-ichi Nuclear Power Plant accident”. Biogeosciences, 6045-6052, 2013.
File đính kèm:
 determination_of_recovery_efficiency_of_137cs_in_seawaterusi.pdf
determination_of_recovery_efficiency_of_137cs_in_seawaterusi.pdf

