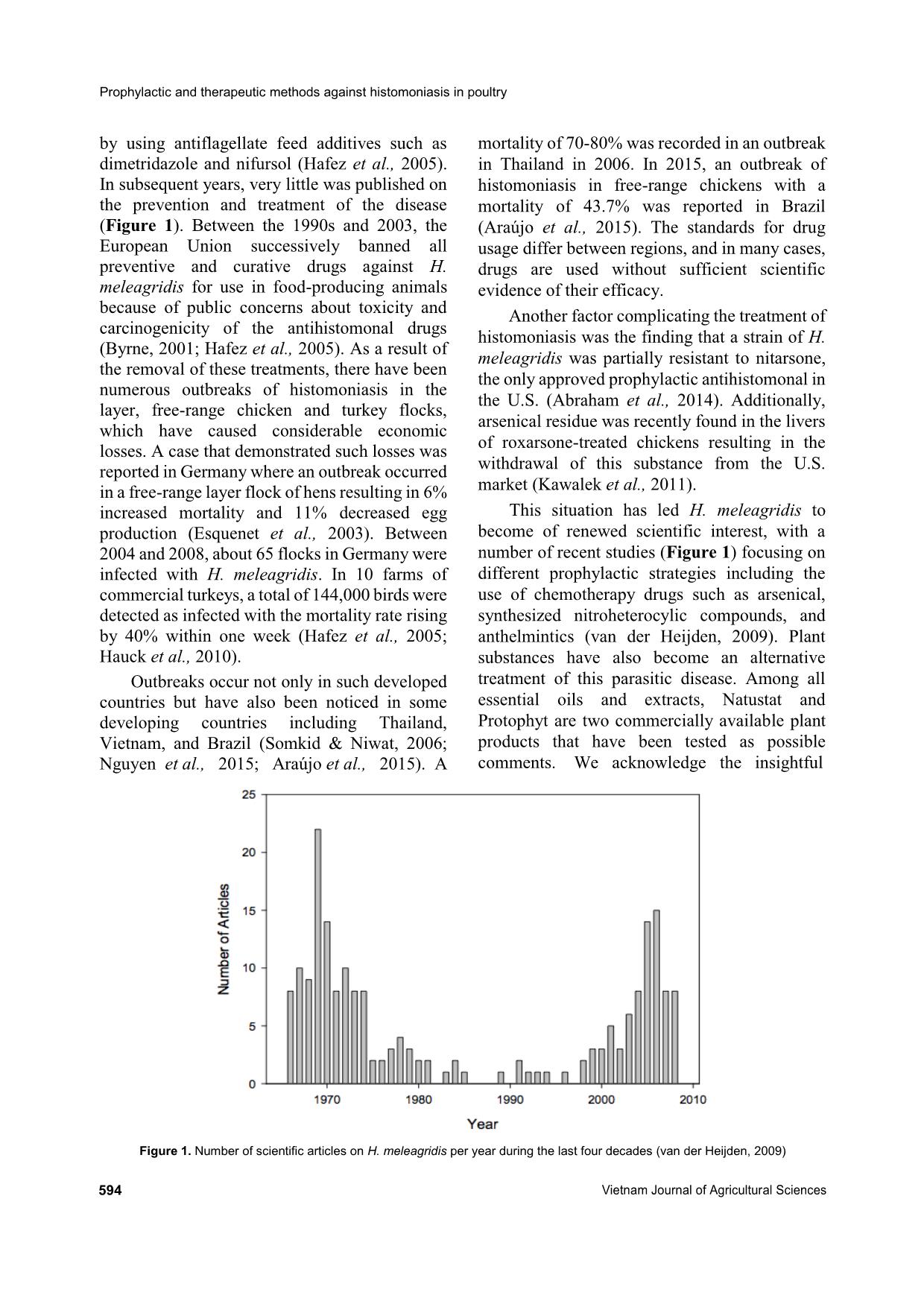
Prophylactic and therapeutic methods against histomoniasis in poultry
Histomoniasis, commonly known as blackhead disease, is a parasitic
disease in poultry caused by the protozoan Histomonas meleagridis.
The availability of various compounds for chemotherapy in the 1970s
resulted in the successful control of blackhead disease. Since the ban
of antihistomonal drugs in the European Union, the disease has reemerged, resulting in up to 100% mortality in turkey flocks. This has
renewed the interest of scientists with numerous publications
focusing on prophylactic strategies. This review summarizes the
literature on the preventive and curative options for the control and
treatment of histomoniasis. Two main approaches to the prophylaxis
of the disease were found, which included chemotherapies and plant
substrate products. Histostat-50 and paromomycin were the only
available drugs that showed antihistomonal activity despite some
concern about their threat to human health and antibiotic resistance.
None of the plant substrate products provided potential protection to
birds against blackhead disease. The use of attenuated histomonads
could be an alternative for the prevention of the disease, but the
production of this vaccine prototype is still challenging due to
advanced technique requirements.

Trang 1
Trang 2

Trang 3

Trang 4

Trang 5

Trang 6

Trang 7

Trang 8
Trang 9

Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Prophylactic and therapeutic methods against histomoniasis in poultry

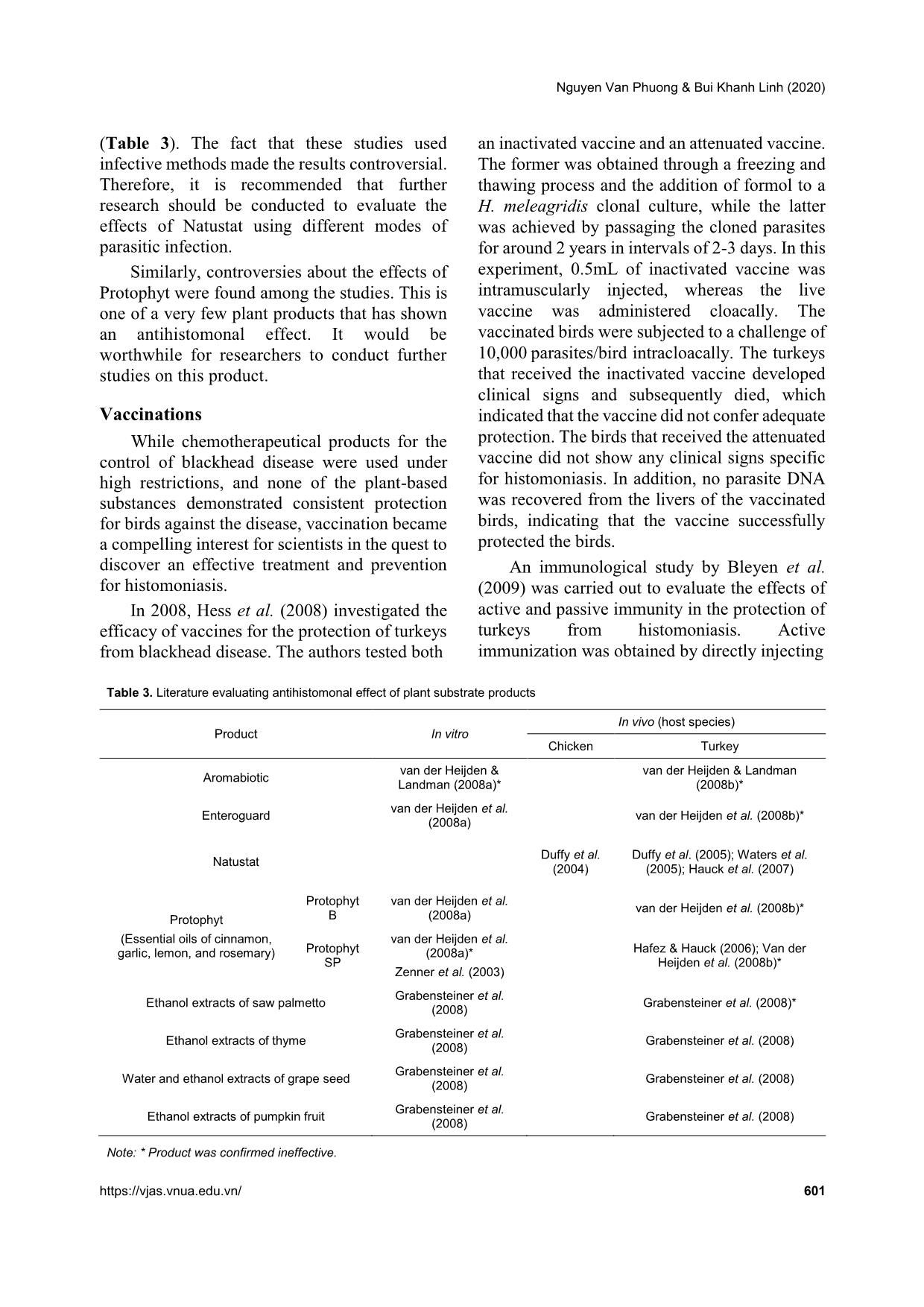
. This indicated that attenuated H. meleagridis did not cause any adverse effects to infected chickens and turkeys. This was one of a very few studies focusing on vaccinations of this parasite in chickens. These findings could be useful for further investigations in the vaccine development for histomoniasis. However, the design of this study was not strongly convincing. The authors used a dose of only 104 histomonads per bird, which was significantly lower than the dose used by other scientists, ranging from 1.4x105 to 106 parasites per bird (Zenner et al., 2003; Hu & McDougald, 2004; Hafez & Hauck, 2006; van Der Heijden et al., 2008). It could explain why no changes were observed in the birds infected by attenuated parasites. Further studies are required to confirm the effectiveness of the immunization produced by these attenuated isolates. A recent study conducted by Nguyen Pham et al. (2013) examined the protective effectiveness of the low-virulent H. meleagridis against blackhead disease in turkeys. The low- virulent H. meleagridis was produced by serial intracloacal passages, which are also known as back passages. The back passage comprised of an intracloacal inoculation of histomonad to 3- week-old birds. Thirteen days post-inoculation, these birds were euthanized to obtain the parasite for reinoculation of new birds. An examination of the virulence of the back passages showed that the last three intracloacal passages, did not cause any of the typical lesions found in turkeys. Subsequently, back passage 10 was used to test the protective capacity against H. meleagridis. Nguyen Van Phuong & Bui Khanh Linh (2020) https://vjas.vnua.edu.vn/ 603 The birds vaccinated with this low virulent back passage yielded minor clinical signs, and lower liver and caecal lesion scores compared to the unvaccinated challenged group; and no mortality compared to 71% in the unvaccinated group in an experimental challenge with a virulent strain of the parasite. This result indicated that the low- virulent H. meleagridis attenuated by serial intracloacally passages was able to protect turkeys from a virulent strain of this parasite. A logical experiment design used in this study made its findings persuasive. In general, most studies evaluating histomoniasis vaccination were conducted exclusively in turkeys. The transfer of serum antibodies from experimentally infected birds was unable to protect birds from histomonads infection, while active immunization created by injecting antigens showed protective activity. Several authors proved that a vaccination with in vitro attenuated parasites could provide protection to turkeys against H. meleagridis independently of administration (Table 4). Additionally, this type of vaccination did not alter the performance of the subjected birds. In a practical sense, the production of an effective vaccine would require not only sophisticated techniques but also a harmonization between laboratory experiments and field demand. The future of histomoniasis treatments Following the ban of nitroimidazoles, recognized as high potential antihistomonal drugs in the European Union and some other countries, due to the concern of ecological and human health threats, researchers were forced to search for viable alternatives in preventing histomoniasis. In general, three different approaches were investigated to deal with the situation. These were the application of antibiotics showing antiprotozoal activity, testing plant substances, particularly some essential oils, and the attempt to develop effective vaccines. Among the various drugs tested, Histostat- 50 and Paromomycin are the two available drugs in some markets that possess antihistomonal activity despite recently raised concerns about deleterious side effects such as bacterial resistance and consumer safety. It is, therefore, crucial for policymakers to strike a balance between the benefits of the consumer and the sustainability of animal production. A large number of plant substances were tested for their effectiveness against histomoniasis, none of which conferred reliable protection to birds in in vivo experiments. There was a lack of standardized methodology for infection among the studies that led to inconsistent results. It is therefore recommended to address this issue in further investigations. Approaches to different types of immunizations showed that only vaccinations with attenuated parasites could induce reliable protection from histomoniasis in turkeys. However, the production of vaccines, particularly for this protist, would be challenging due to a high level of sophisticated techniques required. Acknowledgements We are grateful for the comments of Dr. Anne Beasley at the University of Queensland. She also answered numerous questions about the language of this paper. We also thank Prof. Nguyen Van Tho and Dr. Nguyen Thi Hong Chien, our colleagues at the Vietnam National University of Agriculture, who also looked over our transcriptions and provided valuable comments. Table 4. Literature evaluating the effectiveness of vaccinations in the protection of birds from histomoniasis Passive vaccination Active vaccination Antigens Inactivated parasite Attenuated parasite Bleyen et al. (2009)* Bleyen et al. (2009) Hess et al. (2008)* Hess et al. (2008); Liebhart et al. (2010); Nguyen Pham et al. (2013) Note: * Proved to be ineffective to protect birds from blackhead disease. Prophylactic and therapeutic methods against histomoniasis in poultry 604 Vietnam Journal of Agricultural Sciences We acknowledge the insightful comments offered by the anonymous reviewers and the hard work of the language reviewers who saved us from many errors. References Abraham M., McDougald L. R. & Beckstead R. B. (2014). Blackhead disease: Reduced sensitivity of Histomonas meleagridis to nitarsone in Vitro and in Vivo. Avian diseases. 58(1): 60-63. Administration USFaD. FDA. (2015). Announces Pending Withdrawal of Approval of Nitarsone. Retrieved from https://www.ngfa.org/news/feed-news/fda-announces- pending-withdrawal-of-nitarsone-for-use-in-turkeys- chicken/#:~:text=The%20Food%20and%20Drug%20 Administration,by%20the%20end%20of%202015 on October 10, 2019. Araújo J. L., Olinda R. G., Frade M. T. S., Maia L. Â. & Dantas A. F. M. (2015). Histomoniasis outbreak in free-range chickens in semiarid Paraíba, Brazil. Semina: Ciências Agrárias. 36(1): 307-312. Bleyen N., De Gussem K., Nguyen Pham Anh Dao, Ons E., Van Gerven N. & Goddeeris B. M. (2009). Non- curative, but prophylactic effects of paromomycin in Histomonas meleagridis-infected turkeys and its effect on performance in non-infected turkeys. Veterinary Parasitology. 165(3-4): 248-255. Bleyen N., Ons E., De Gussem M. & Goddeeris B. M. (2009). Passive immunization against Histomonas meleagridis does not protect turkeys from an experimental infection. Avian Pathology. 38(1): 71-76. Byrne D. (2001). Commission Regulation (EC) No. 2205/2001 of November 14, 2001 amending Council Directive 70/524/EEC concerning additives in feedingstuffs as regards withdrawal of the authorisation of certain additives 2001. Retrieved from https://eur-lex.europa.eu/legal- content/EN/TXT/?uri=CELEX%3A32001R2205 on October 10, 2019. Cushman S. (1893). The production of turkeys. Rhode Island Agricultural Experiment Station Bulletin. 25: 89-123. Duffy C. F., Sims M. D. & Power R. F. (2005). Evaluation of dietary Natustat for control of Histomonas meleagridis in male turkeys on infected litter. Avian Diseases. 49(3):423-425. Duffy C. F., Sims M. D. & Power R. F. (2004). Preliminary evaluation of dietary natustat™ versus histostat® (Nitarsone) for control of Histomonas meleagridis in broiler chickens on infected litter. International Journal of Poultry Science. 3(12): 753-757. EFSA (2009). Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the preliminary evaluation of the safety and efficacy of paromomycin sulphate for turkeys for fattening and turkeys reared for breeding. The EFSA Journal. 1095: 1-22. Esquenet C., De Herdt P., De Bosschere H., Ronsmans S., Ducatelle R. & Van Erum J. (2003). An outbreak of histomoniasis in free-range layer hens. Avian Pathology. 32(3): 305-308. Grabensteiner E., Liebhart D., Arshad N. & Hess M. (2008) Antiprotozoal activities determined in vitro and in vivo of certain plant extracts against Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. Parasitology Research. 103(6): 1257- 1264. Grabensteiner E., Liebhart D., Weissenböck H. & Hess M. (2006). Broad dissemination of Histomonas meleagridis determined by the detection of nucleic acid in different organs after experimental infection of turkeys and specified pathogen-free chickens using a mono-eukaryotic culture of the parasite. Parasitology International. 55(4): 317-322. Hafez H. M., Schulze D., Hauck R. & Lüschow D. (2005). Histomonas meleagridis: The Situation after the Ban of the Last Available Drug in the EU. Proceedings of the fifty-fourth Western pounltry disease conference. Vancouver, B.C., Canada. Hafez H.M., Hauck R., Gad W., De Gussem K. & Lotfi A. (2010). Pilot study on the efficacy of paromomycin as a histomonostatic feed additive in turkey poults experimentally infected with Histomonas meleagridis. Archives of Animal Nutrition. 64(1): 77-84. Hafez H. M. & Hauck R. (2006). Efficacy of a herbal product against Histomonas meleagridis after experimental infection of turkey poults. Archives of Animal Nutrition. 60(5): 436-642. Hauck R., Armstrong P. L., Fuller L. & McDougald L. R. (2007). The effect of Natustat® on Histomoniasis in turkeys. Proceedings of the 56th Western poultry disease . March 26-29, 2007. Las Vegas, Nevada. 102- 104. Hauck R., Balczulat S. & Hafez H. M. (2010). Detection of DNA of Histomonas meleagridis and Tetratrichomonas gallinarum in German poultry flocks between 2004 and 2008. Avian Diseases. 54(3): 1021-1025. Hess M., Liebhart D., Grabensteiner E. & Singh A. (2008). Cloned Histomonas meleagridis passaged in vitro resulted in reduced pathogenicity and is capable of protecting turkeys from histomonosis. Vaccine. 26(33): 4187-4193. Hu J. & McDougald L. R. (2004). The efficacy of some drugs with known antiprotozoal activity against Histomonas meleagridis in chickens. Veterinary parasitology. 121(3-4): 233-238. Kawalek J. C., Carson M., Conklin S., Lancaster V., Howard K. & Ward J. (2011). Provide data on various arsenic species present in broilers treated with Nguyen Van Phuong & Bui Khanh Linh (2020) https://vjas.vnua.edu.vn/ 605 roxarsone: comparison with untreated birds. Final report on Study 275.30. 2011. US Food and Drug Administration: Laurel, MD, USA. Kempf I., Le Roux A., Perrin-Guyomard A., Mourand G., Le Devendec L., Bougeard S., Richez P., Le Pottier G. & Eterradossi N. (2013). Effect of in-feed paromomycin supplementation on antimicrobial resistance of enteric bacteria in turkeys. The Veterinary Journal. 198(2): 398-403. DOI: 10.1016/j.tvjl.2013.05.030. Liebhart D., Windisch M. & Hess M. (2010). Oral vaccination of 1-day-old turkeys with in vitro attenuated Histomonas meleagridis protects against histomonosis and has no negative effect on performance. Avian Pathology. 39(5): 399-403. Lund E. E. & Burtner Jr R. H. (1957). Infectivity of Heterakis gallinae eggs with Histomonas meleagridis. Experimental Parasitology. 6(2):189-93. DOI: 10.1016/0014-4894(57)90014-0. McDougald L. R. & Fuller L. (2005). Blackhead disease in turkeys: direct transmission of Histomonas meleagridis from bird to bird in a laboratory model. Avian Diseases. 49(3): 328-331. McDougald L. R. & Hu J. (2001). Blackhead disease (Histomonas meleagridis) aggravated in broiler chickens by concurrent infection with cecal coccidiosis (Eimeria tenella). Avian Diseases. 45(2): 307-312. Nguyen Duc Tan, Bilic I., Jaskulsa B., Hess M. B., Le Duc Quyet, Le Hua Ngoc Luc, Huynh Vy Vu, Nguyen Thi Sam & Vu Khac Hung (2015). Prevalence and genetic characterization of Histomonas meleagridis in chickens in Vietnam. Avian diseases. 59(2):309-314. DOI: 10.1637/10964-102414-Reg. Nguyen Pham Anh Dao., De Gussem J. K. & Goddeeris B. M. (2013). Intracloacally passaged low-virulent Histomonas meleagridis protects turkeys from histomonosis. Veterinary Parasitology. 196(3): 307-313. Reece R. L., Beddome V. D. & Barr D. A. (1986). Diseases diagnosed in replacement layer and breeder chicken flocks in Victoria, Australia, 1977 to 1985. Veterinary Record. 119(19): 471-475. Somkid K. P. K. & Niwat C. (2006). Outbreak of histomoniasis in cross breed native chickens from one private farm in Nakorn Pathom province. Retrieved on October 18, 2019 from search/search.do?recordID=TH2006000328. Swayne DEG. J. R., McDougald L. R., Nolan L. K., Suarez D. L. & Nair V. (2013). Diseases of Poultry. 13, editor: John Wiley & Sons, Inc; 1423. van der Heijden H. M. J. F., De Gussem K. & Landman W. J. M. (2011). Assessment of the antihistomonal effect of paromomycin and tiamulin. Tijdschrift Voor Diergeneeskunde. 136(6): 410-416. van der Heijden H. M. (2009). Detection, typing and control of Histomonas meleagridis. Retrieved from 42/heijden.pdf?sequence=1 on October 18, 2019. van der Heijden H. M. J. F. & Landman W. J. M. (2008a). In vitro effect of herbal products against Histomonas meleagridis. Veterinary Parasitology. 154(1): 1-7. van der Heijden H. M. & Landman W. J. M. (2008b). In vivo effect of herbal products against Histomonas meleagridis in turkeys. Avian Pathology: journal of the WVPA. 37(1): 45-50. Waters S. M., Duffy C. F. & Power R. F. G. (2005). PCR- DGGE analysis of caecal microflora of Natustat™ supplemented turkeys challenged with Histomonas meleagridis. International Journal of Poultry Science. 4(9): 620-627. Zahoor M. A., Liebhart D. & Hess M. (2011). Progression of histomonosis in commercial chickens following experimental infection with an in vitro propagated clonal culture of Histomonas meleagridis. Avian Diseases. 55(1): 29-34. DOI: 10.1637/9508-082110-Reg.1. Zenner L., Callait M. P., Granier C. & Chauve C. (2003). In vitro effect of essential oils from Cinnamomum aromaticum, Citrus limon and Allium sativum on two intestinal flagellates of poultry, Tetratrichomonas gallinarum and Histomonas meleagridis. Parasite. 10(2): 153-157. DOI: 10.1051/parasite/2003102153.
File đính kèm:
 prophylactic_and_therapeutic_methods_against_histomoniasis_i.pdf
prophylactic_and_therapeutic_methods_against_histomoniasis_i.pdf

