Influence of grain size on the electrochemical properties of LaNi3.55Al0.3Mn0.4Co0.75 compound use for ni-Mh battery
The production of Ni-MH commercial batteries extensively used in cordless appliances such as video-cameras, lap-top computers and cellular telephones because it does
not contain poisonous heavy metals, has higher energy density (55 – 65 wh/kg), longer
cycle life than the Ni-Cd battery and lower price. To improve energy density, cycle life,
current density. many studies have been carried out. The alloys of TiFe, Mg2Ni, LaNi5
were pulverized into a powder with a mean diameter of nano meter only by mechanical
milling method [1,3]. M.Jurczyka et al [1] and V.X. Thang et al [3] showed that the energy
capacity rapidly in creased, the life time of the electrode also longer when the grain size
of the alloys reduces to nano met scale. Z.Chen et al. [2] studies AB5 alloys mechanical
milling to reduce grain size to nano met rouge and realized that the life time of battery
increase.
In this work, the influence of the grain size on the electrochemical properties of the
LaNi3.55Al0.3Mn0.4Co0.75 compound were investigated and the obtained results were also
discussed.

Trang 1

Trang 2
Trang 3
Trang 4

Trang 5

Trang 6
Tóm tắt nội dung tài liệu: Influence of grain size on the electrochemical properties of LaNi3.55Al0.3Mn0.4Co0.75 compound use for ni-Mh battery

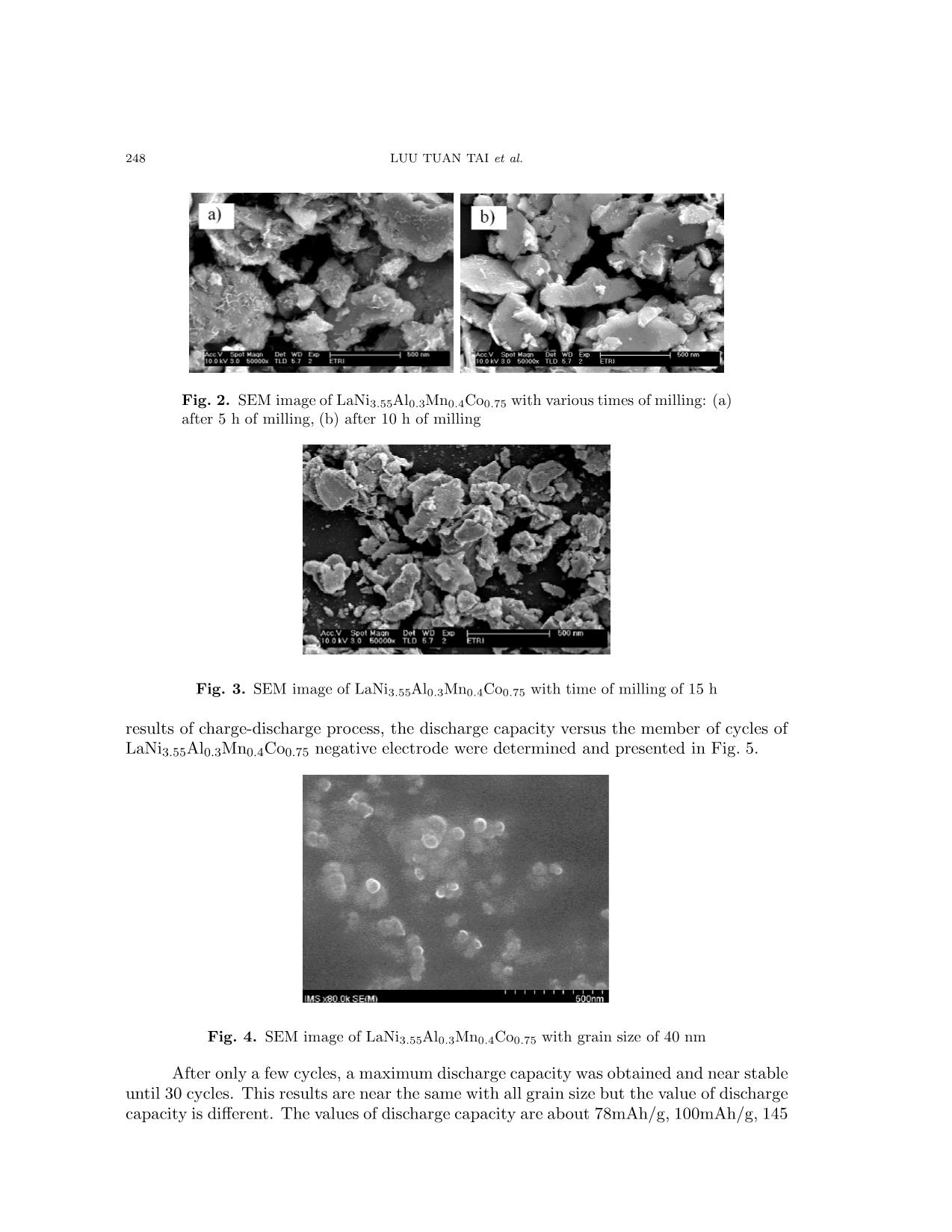
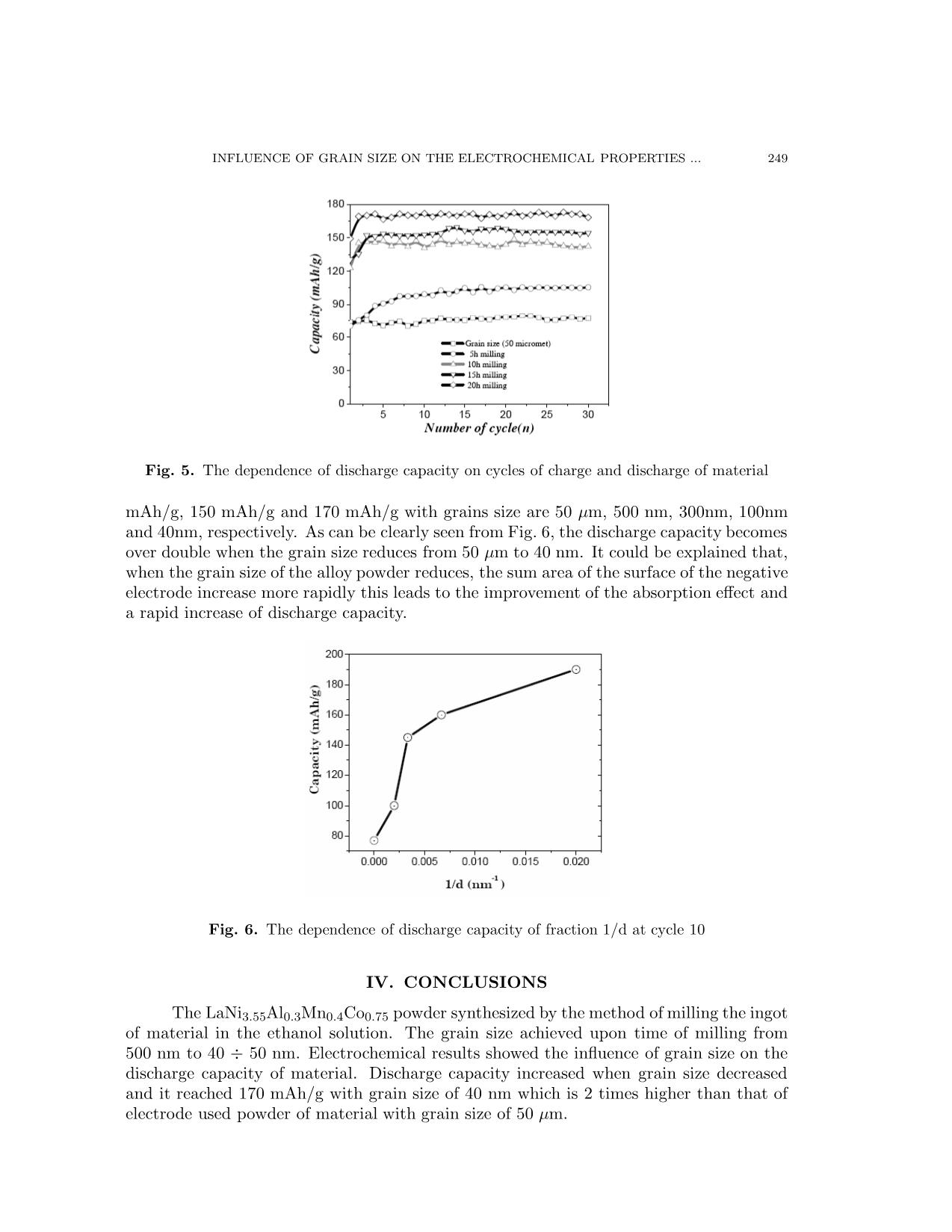
Communications in Physics, Vol. 17, No. 4 (2007), pp. 246-250 INFLUENCE OF GRAIN SIZE ON THE ELECTROCHEMICAL PROPERTIES OF LaNi3.55Al0.3Mn0.4Co0.75 COMPOUND USE FOR NI-MH BATTERY LUU TUAN TAI Faculty of Physics, College of Science, Vietnam National University, Hanoi TRAN BAO TRUNG, THAN DUC HIEN International Training Institute for Materials Science (ITIMS) LUU HOAI NAM Faculty of Engineering Physics and Nano Technology, College of Applied Science and Technology, Vietnam National University, Hanoi Abstract. In this work, LaNi3.55Al0.3Mn0.4Co0.75 compounds were prepared by arc melting method in the Ar atmosphere. The structure and grain size were tested by X-ray diffraction and TEM measurements. Electrochemical properties and battery parameter were carried out by bipotentio- stat and battery tester equipments. The results show that with the grain size 50µm, capacity of the negative electrode was 77 mAh/g. When grain size of LaNi3.55Al0.3Mn0.4Co0.75 powder use for negative electrode reduces to 40nm, the capacity rapidly increase to 170 mAh/g and other battery parameter also improve. I. INTRODUCTION The production of Ni-MH commercial batteries extensively used in cordless appli- ances such as video-cameras, lap-top computers and cellular telephones because it does not contain poisonous heavy metals, has higher energy density (55 – 65 wh/kg), longer cycle life than the Ni-Cd battery and lower price. To improve energy density, cycle life, current density... many studies have been carried out. The alloys of TiFe, Mg2Ni, LaNi5 were pulverized into a powder with a mean diameter of nano meter only by mechanical milling method [1,3]. M.Jurczyka et al [1] and V.X. Thang et al [3] showed that the energy capacity rapidly in creased, the life time of the electrode also longer when the grain size of the alloys reduces to nano met scale. Z.Chen et al. [2] studies AB5 alloys mechanical milling to reduce grain size to nano met rouge and realized that the life time of battery increase. In this work, the influence of the grain size on the electrochemical properties of the LaNi3.55Al0.3Mn0.4Co0.75 compound were investigated and the obtained results were also discussed. II. EXPERIMENTS The samples LaNi3.55Al0.3Mn0.4Co0.75 were prepared by are melting method under argon gas atmosphere. The starting materials of purity of at least 99,9% were weighted based on their compositions. After grinding, the ingots of alloy powder with grain size about 50µm. INFLUENCE OF GRAIN SIZE ON THE ELECTROCHEMICAL PROPERTIES ... 247 This powder was pulverized in ethanol solution by planet pulverijeter with 250 cycles per minute of velocity and various times 5 h, 10 h, 15 h and 20 hours. The crystal structure and grain size were examined by X-ray diffraction (XRD) and SEM. For the electrochem- ical measurements, negative electrodes were prepared by mixing LaNi3.55Al0.3Mn0.4Co0.75 powder with cooper and nickel powders and binder in 70:28:2 weight ratio. This mixture was pressed onto a nickel mesh at a pressure of 6 Tons/cm2. The positive electrode used was Na(OH)2 and the electrolyte was either 6M KOH or 1M LiOH in 6M KOH. The pur- pose of LiOH addition into the 6M KOH electrolyte is to increase electrochemical activity of the MH electrode. Electrochemical measurement were carried out after full charge/discharge activation with constant charge current of 50mA/g by battery tester equipment. III. RESULTS AND DISCUSSION Fig. 1 are the results of X-ray diffraction patterns of LaNi3.55Al0.3Mn0.4Co0.75 pow- ders with various milling time. The X-ray diffraction pattern of the rough grinding powder shows that this sample is crystallized in the hexagonal CaCu5-type structure and single phase. The comparisons of X-ray diffraction pattern between three type powders: rough grinding, 15 h grinding and 20 h grinding show that the grain size reduces by grinding time in crease. Fig. 1. The results of X-ray diffraction patterns of LaNi3.55Al0.3Mn0.4Co0.75 pow- ders with various milling time Mean grain size of the powder was determined by SEM and TEM pictures. Fig. 2 and Fig. 3 are SEM pictures of LaNi3.55Al0.3Mn0.4Co0.75 sample with various grinding time 5, 10, and 15 hours respectively. It is clearly that when the grinding time increase, the grain size decrease quickly. After 5h, 10h and 15h milling times the grain size of the powders are 500nm, 300nm and about 150 nm, respectively. The grain size reduced to about 40nm after 20 h milling see Fig. 4. The influence of the grain size on the electrochemical properties of the material powder was investigated by charge/discharge cycles with constant current of 50 mA/g during 8 hours. The rest time between charge and discharge cycles is 30 minutes. Before electrochemical measurement, the training galvanostatic charge-discharge process was per- formed with a current of 20 mA for 1 hour. With 10 cycles to put electrode in stable. From 248 LUU TUAN TAI et al. Fig. 2. SEM image of LaNi3.55Al0.3Mn0.4Co0.75 with various times of milling: (a) after 5 h of milling, (b) after 10 h of milling Fig. 3. SEM image of LaNi3.55Al0.3Mn0.4Co0.75 with time of milling of 15 h results of charge-discharge process, the discharge capacity versus the member of cycles of LaNi3.55Al0.3Mn0.4Co0.75 negative electrode were determined and presented in Fig. 5. Fig. 4. SEM image of LaNi3.55Al0.3Mn0.4Co0.75 with grain size of 40 nm After only a few cycles, a maximum discharge capacity was obtained and near stable until 30 cycles. This results are near the same with all grain size but the value of discharge capacity is different. The values of discharge capacity are about 78mAh/g, 100mAh/g, 145 INFLUENCE OF GRAIN SIZE ON THE ELECTROCHEMICAL PROPERTIES ... 249 Fig. 5. The dependence of discharge capacity on cycles of charge and discharge of material mAh/g, 150 mAh/g and 170 mAh/g with grains size are 50 µm, 500 nm, 300nm, 100nm and 40nm, respectively. As can be clearly seen from Fig. 6, the discharge capacity becomes over double when the grain size reduces from 50 µm to 40 nm. It could be explained that, when the grain size of the alloy powder reduces, the sum area of the surface of the negative electrode increase more rapidly this leads to the improvement of the absorption effect and a rapid increase of discharge capacity. Fig. 6. The dependence of discharge capacity of fraction 1/d at cycle 10 IV. CONCLUSIONS The LaNi3.55Al0.3Mn0.4Co0.75 powder synthesized by the method of milling the ingot of material in the ethanol solution. The grain size achieved upon time of milling from 500 nm to 40 ÷ 50 nm. Electrochemical results showed the influence of grain size on the discharge capacity of material. Discharge capacity increased when grain size decreased and it reached 170 mAh/g with grain size of 40 nm which is 2 times higher than that of electrode used powder of material with grain size of 50 µm. 250 LUU TUAN TAI et al. AKNOWLEDGEMENTS This works is supported by the Vietnam National University research program under grant QG-07-04 REFERENCES [1] M. Jurczyka, L. Smardzb, M. Makowiecka, E. Jankowska, K. Smardz, Journal of Physics and Chem- istry of Solids, 65 (2004) 545-548 [2] Z. Chen, Y. Su, M. Lu, D. Zhou, P. Huang, Materials Research Bulletin, 33 (10) (1998) 1449 [3] Vu Xuan Thang, Than Duc Hien, Luu Tuan Tai, Nguyen Phuc Duong, Proceedings of the 4th National Conference of Physics, Hanoi (November 2005), Vietnam. Received 06 September 2007.
File đính kèm:
 influence_of_grain_size_on_the_electrochemical_properties_of.pdf
influence_of_grain_size_on_the_electrochemical_properties_of.pdf

