Growth and physiological responses of sugarcane to drought stress at an early growth stage
A pot experiment was conducted in a net house to evaluate the effects
of drought stress (a 20-day water withholding treatment from 100-
120 days after planting) on the growth and physiology of five
sugarcane cultivars. The results showed that water stress at an early
stage significantly affected sugarcane growth and physiology.
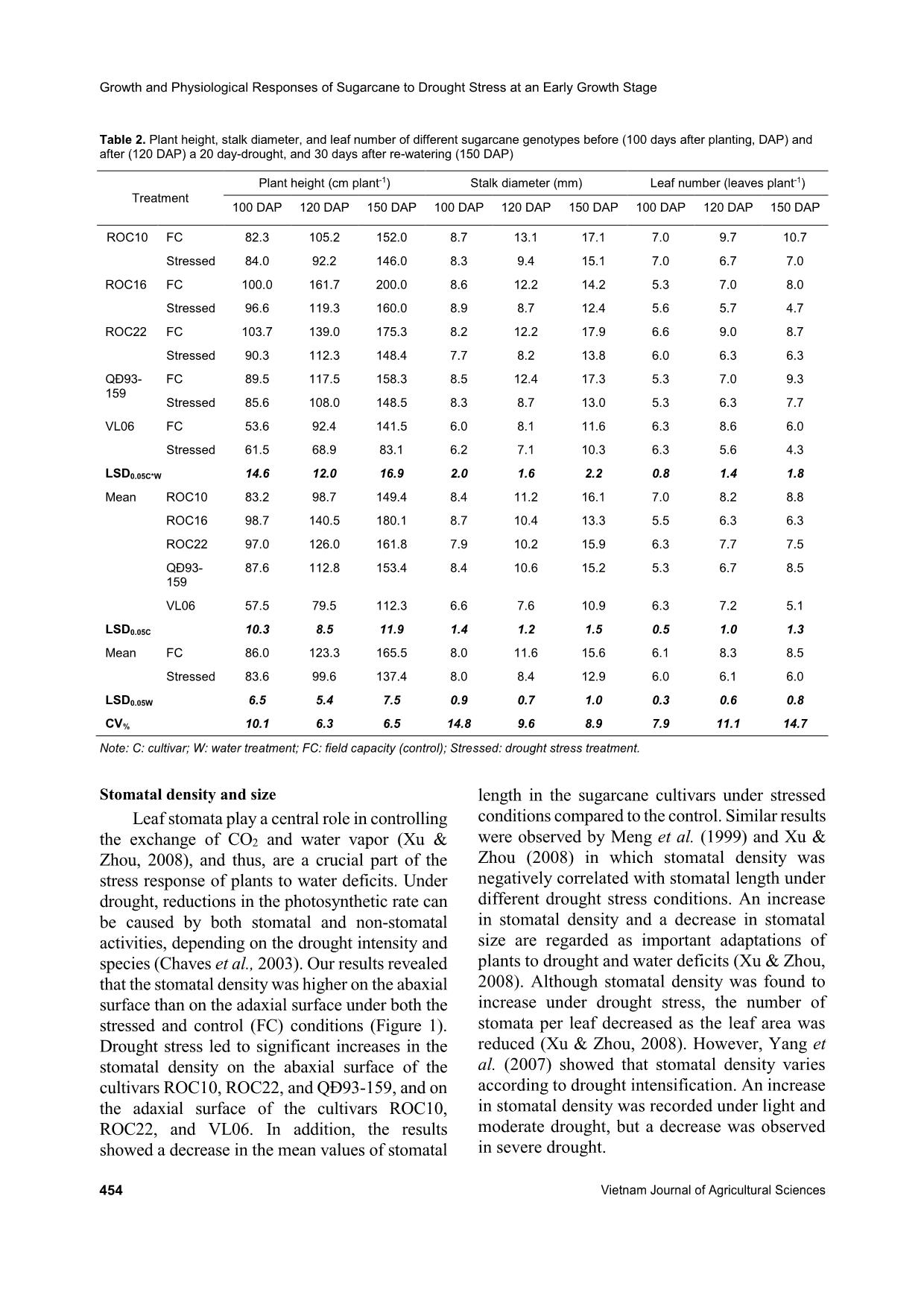
Water stress resulted in reductions in plant height, stalk diameter,
and leaf number of sugarcane, in addition to reductions in the
photosynthetic pigment content, Fv/Fm, and SPAD (Soil Plant
Analysis Development) readings after the 20-day withholding
water period (120 DAP), and in stem, root, and leaf fresh weights,
and leaf area at 150 DAP. Besides, drought stress led to increases
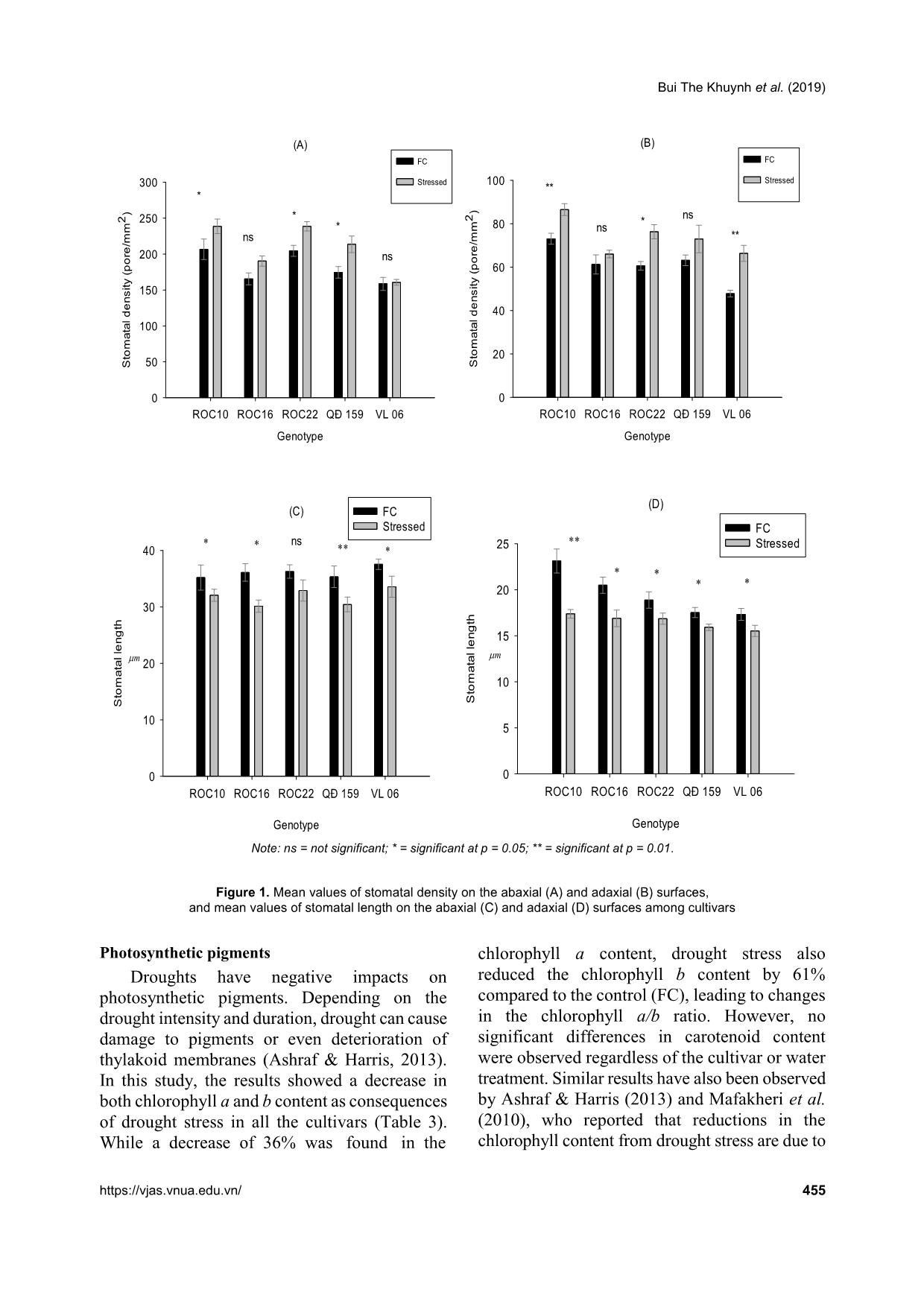
in stomata density and decreases in stomata length. Variation was
also found among the cultivars in response to water stress.
Significant genotypic differences in stem fresh weight and leaf
area under water stress among the cultivars were observed. The
highest value of stem fresh weight under stressed conditions was
recorded in ROC22 (50.6g), followed by QĐ159 (46.5g), ROC16
(46.2g), ROC10 (46.1g), and VL06 (44.4g). However, the highest
DTI was recorded in ROC16, followed by VL06, ROC10, QĐ93-
159, and ROC22, respectively.

Trang 1

Trang 2

Trang 3
Trang 4
Trang 5
Trang 6
Trang 7
Trang 8

Trang 9

Trang 10
Tóm tắt nội dung tài liệu: Growth and physiological responses of sugarcane to drought stress at an early growth stage

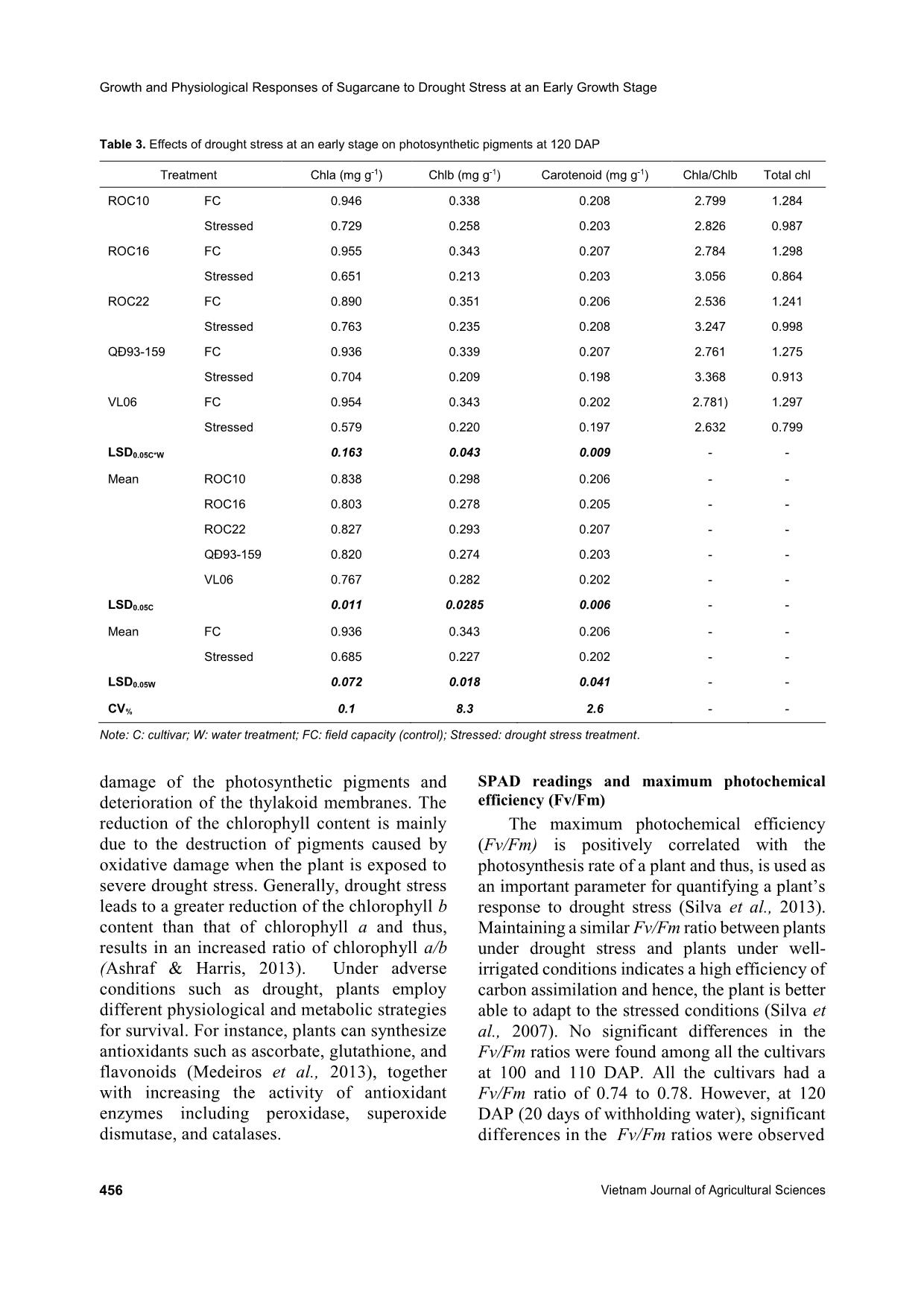
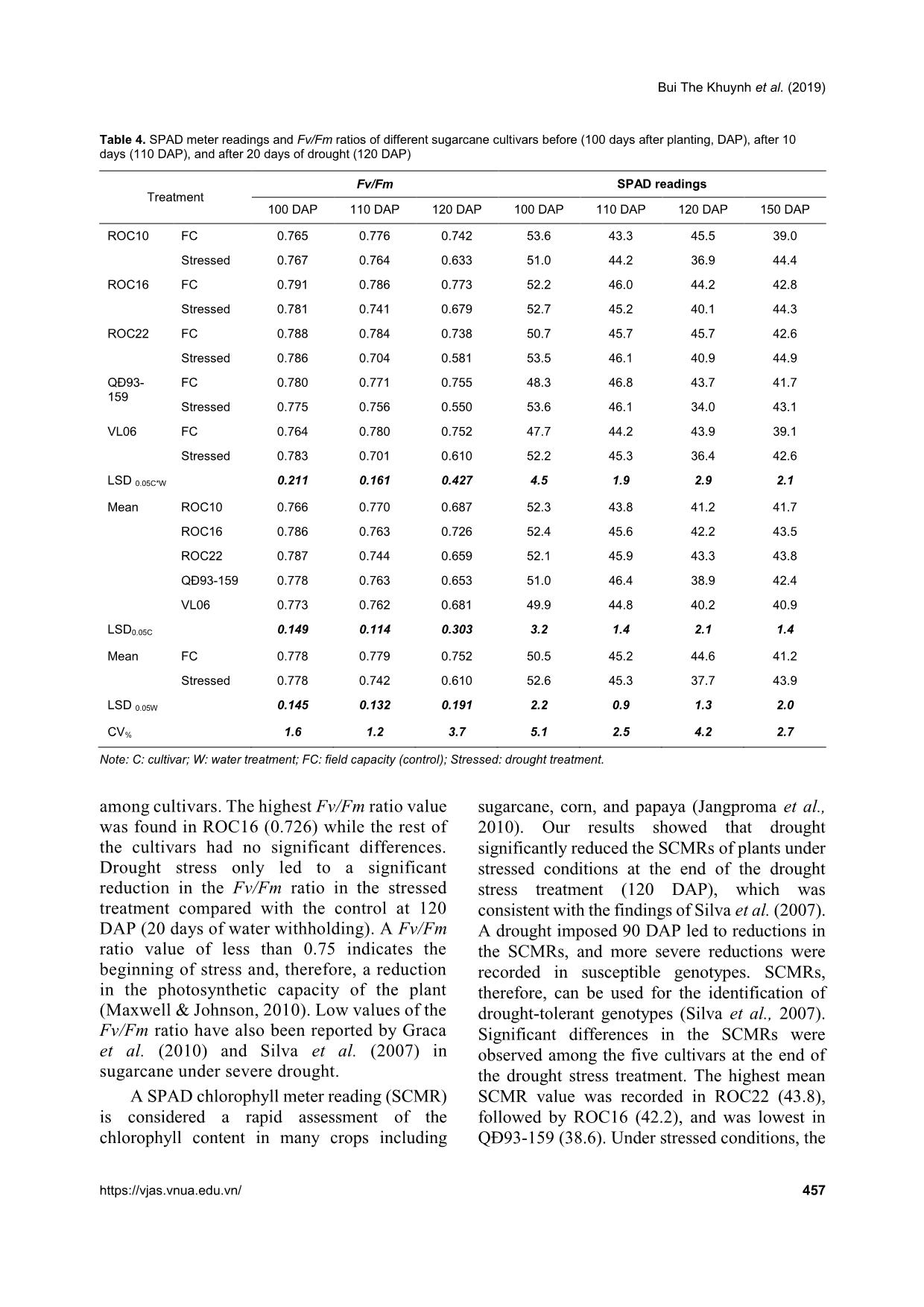
olic strategies for survival. For instance, plants can synthesize antioxidants such as ascorbate, glutathione, and flavonoids (Medeiros et al., 2013), together with increasing the activity of antioxidant enzymes including peroxidase, superoxide dismutase, and catalases. SPAD readings and maximum photochemical efficiency (Fv/Fm) The maximum photochemical efficiency (Fv/Fm) is positively correlated with the photosynthesis rate of a plant and thus, is used as an important parameter for quantifying a plant’s response to drought stress (Silva et al., 2013). Maintaining a similar Fv/Fm ratio between plants under drought stress and plants under well- irrigated conditions indicates a high efficiency of carbon assimilation and hence, the plant is better able to adapt to the stressed conditions (Silva et al., 2007). No significant differences in the Fv/Fm ratios were found among all the cultivars at 100 and 110 DAP. All the cultivars had a Fv/Fm ratio of 0.74 to 0.78. However, at 120 DAP (20 days of withholding water), significant differences in the Fv/Fm ratios were observed Bui The Khuynh et al. (2019) https://vjas.vnua.edu.vn/ 457 Table 4. SPAD meter readings and Fv/Fm ratios of different sugarcane cultivars before (100 days after planting, DAP), after 10 days (110 DAP), and after 20 days of drought (120 DAP) Treatment Fv/Fm SPAD readings 100 DAP 110 DAP 120 DAP 100 DAP 110 DAP 120 DAP 150 DAP ROC10 FC 0.765 0.776 0.742 53.6 43.3 45.5 39.0 Stressed 0.767 0.764 0.633 51.0 44.2 36.9 44.4 ROC16 FC 0.791 0.786 0.773 52.2 46.0 44.2 42.8 Stressed 0.781 0.741 0.679 52.7 45.2 40.1 44.3 ROC22 FC 0.788 0.784 0.738 50.7 45.7 45.7 42.6 Stressed 0.786 0.704 0.581 53.5 46.1 40.9 44.9 QĐ93- 159 FC 0.780 0.771 0.755 48.3 46.8 43.7 41.7 Stressed 0.775 0.756 0.550 53.6 46.1 34.0 43.1 VL06 FC 0.764 0.780 0.752 47.7 44.2 43.9 39.1 Stressed 0.783 0.701 0.610 52.2 45.3 36.4 42.6 LSD 0.05C*W 0.211 0.161 0.427 4.5 1.9 2.9 2.1 Mean ROC10 0.766 0.770 0.687 52.3 43.8 41.2 41.7 ROC16 0.786 0.763 0.726 52.4 45.6 42.2 43.5 ROC22 0.787 0.744 0.659 52.1 45.9 43.3 43.8 QĐ93-159 0.778 0.763 0.653 51.0 46.4 38.9 42.4 VL06 0.773 0.762 0.681 49.9 44.8 40.2 40.9 LSD0.05C 0.149 0.114 0.303 3.2 1.4 2.1 1.4 Mean FC 0.778 0.779 0.752 50.5 45.2 44.6 41.2 Stressed 0.778 0.742 0.610 52.6 45.3 37.7 43.9 LSD 0.05W 0.145 0.132 0.191 2.2 0.9 1.3 2.0 CV% 1.6 1.2 3.7 5.1 2.5 4.2 2.7 Note: C: cultivar; W: water treatment; FC: field capacity (control); Stressed: drought treatment. among cultivars. The highest Fv/Fm ratio value was found in ROC16 (0.726) while the rest of the cultivars had no significant differences. Drought stress only led to a significant reduction in the Fv/Fm ratio in the stressed treatment compared with the control at 120 DAP (20 days of water withholding). A Fv/Fm ratio value of less than 0.75 indicates the beginning of stress and, therefore, a reduction in the photosynthetic capacity of the plant (Maxwell & Johnson, 2010). Low values of the Fv/Fm ratio have also been reported by Graca et al. (2010) and Silva et al. (2007) in sugarcane under severe drought. A SPAD chlorophyll meter reading (SCMR) is considered a rapid assessment of the chlorophyll content in many crops including sugarcane, corn, and papaya (Jangproma et al., 2010). Our results showed that drought significantly reduced the SCMRs of plants under stressed conditions at the end of the drought stress treatment (120 DAP), which was consistent with the findings of Silva et al. (2007). A drought imposed 90 DAP led to reductions in the SCMRs, and more severe reductions were recorded in susceptible genotypes. SCMRs, therefore, can be used for the identification of drought-tolerant genotypes (Silva et al., 2007). Significant differences in the SCMRs were observed among the five cultivars at the end of the drought stress treatment. The highest mean SCMR value was recorded in ROC22 (43.8), followed by ROC16 (42.2), and was lowest in QĐ93-159 (38.6). Under stressed conditions, the Growth and Physiological Responses of Sugarcane to Drought Stress at an Early Growth Stage 458 Vietnam Journal of Agricultural Sciences SCMRs were maintained at an average of 40 in ROC22 and ROC16, which were significantly higher compared to those in ROC10, VL06, and QĐ93-159. This suggests a higher capacity of the ROC22 and ROC16 plants to conserve their photosynthetic pigment content during drought stress. According to Silva et al. (2013), a SCMR below 40 in sugarcane indicates the start of chlorophyll deficiency, which affects the photosynthetic activities of the plants. Thus, the low SCMRs (below 40) recorded in ROC10, VL06, and QĐ93-159 indicate drought stress sensitivities. No significant difference in terms of the SCMRs was found between the control and stressed treatments at 30 days after re-watering (150 DAP). Plant fresh weight, leaf area (LA), and drought tolerance index (DTI) at recovery (150 DAP) Sugarcane plants exposed to prolonged drought have been reported to experience a reduction in growth (Jaiphong et al., 2016). Our results showed that root, leaf, and stem fresh weights, and leaf area were significantly reduced in plants under the drought treatment relative to the control (Table 5). Fresh root weight was reduced by 51.5% compared to the drought stress treatment at 150 DAP. This matches with the findings in early studies by Medeiros et al. (2013), Jaiphong et al. (2016), and Silva et al. (2013). Significant differences in root fresh weight, leaf fresh weight, stem fresh weight, and Table 5. Effects of drought stress at an early stage on the plant fresh weight, leaf area, and drought tolerance index (DTI) of five cultivars at 150 DAP Treatment Root fresh weight (g plant-1) Leaf fresh weight (g plant-1) Stem fresh weight (g plant-1) Leaf area (cm2) Drought tolerance index (DTI) ROC10 FC 57.2 158.6 79.3 18.2 - Stressed 30.7 76.5 46.1 9.5 0.59 ROC16 FC 50 188.8 71.2 22.5 - Stressed 31.3 78.8 46.2 10.5 0.69 ROC22 FC 93.1 176.5 96.7 23 - Stressed 41.3 115.6 50.6 17.1 0.55 QĐ93-159 FC 58.1 164.6 80.5 14.9 - Stressed 21.2 82.5 46.5 8.8 0.57 VL06 FC 46.5 91.3 64.7 17.2 - Stressed 26.7 54.9 44.4 5.7 0.68 LSD 0.05C*W 4.1 10.5 4.4 1.9 Mean ROC10 43.9 117.5 62.7 13.9 ROC16 40.6 133.8 58.7 16.5 ROC22 67.2 146.1 73.6 20.1 QĐ93-159 39.6 123.5 63.5 11.8 VL06 36.6 73.1 54.5 11.4 LSD0.05C 2.8 7.4 3.1 1.3 Mean FC 60.9 155.9 78.5 19.2 Stressed 30.2 81.6 46.8 10.3 LSD 0.05W 1.8 4.7 2 0.9 CV% 5.2 5.2 4.2 7.8 Note: C: cultivar; W: water treatment; FC: field capacity (control); Stressed: drought treatment. Bui The Khuynh et al. (2019) https://vjas.vnua.edu.vn/ 459 leaf area were found among sugarcane cultivars. The mean values of stem fresh weight ranged from 54.5g (in VL06) to 73.6g (in ROC22). Significant differences in stem fresh weight and leaf area were also found among the cultivars under drought stress. The highest value of stem fresh weight under stressed conditions was recorded in ROC22 (50.6g), followed by QĐ93- 159 (46.5g), ROC16 (46.2g), ROC10 (46.1g), and VL06 (44.4g). Leaf area also varied among the sugarcane cultivars and ranged from 11.4cm2 in VL06 to 20.1cm2 in ROC22. A reduction in plant growth under water deficit conditions can be seen as the consequence of the decrease in cell elongation caused by the interruption of water flow from the xylem to surrounding elongation cells, the increase in cell sap concentration, and the dehydration of the protoplasm (Nonami, 1998; Larcher, 2003). Low biomass accumulation of sugarcane, when exposed to drought stress, can be explained by reductions in light interception, plant extension rate, and photosynthetic capacity (Koonjah et al., 2006). The drought tolerance index (DTI) has been used as an important parameter in evaluating the tolerance ability of crops. Among the sugarcane cultivars, the highest DTI was recorded in ROC16, followed by VL06, ROC10, QĐ93-159, and ROC22, respectively. Conclusions Drought stress at an early growth stage (100- 120 DAP) significantly affected the growth and physiological characteristics of five sugarcane cultivars. A 20-day water withholding treatment resulted in reductions in plant height, stalk diameter, and leaf number of sugarcane, in addition to reductions in photosynthetic pigment content, Fv/Fm, and SPAD readings at 120 DAP, and in stem, root, and leaf fresh weights, and leaf area at 150 DAP (30 days after re-watering). Furthermore, drought stress led to reductions in stomatal density and increases in stomatal length. Variation was also found among the cultivars in response to drought stress. The highest value of stem fresh weight under stressed conditions was recorded in ROC22 (50.6g), followed by QĐ93- 159 (46.5g), ROC16 (46.2g), ROC10 (46.1g), and VL06 (44.4g). However, the highest DTI was recorded in ROC16, followed by VL06, ROC10, QĐ93-159, and ROC22, respectively. Conflicts of Interest The authors declare no conflicts of interest. References Amalraj R. S., Selvaraj N., Veluswamy G. K., Rananujan R. P., Muthurajan R. & Palaniyandi M. (2010). Sugarcane proteomics: Establishment of a protein extraction method for 2-DE in stalk tissues and initiation of sugarcane proteome reference map. Electrophoresis. 31: 1959-1974. Arnon D. I. (1949). Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiology. 24(1): 1-15. Ashraf M. & Harris P. J. C. (2013). Photosynthesis under stressful environments: An overview. Photosynthetica. 51(2): 163-190. Begcy K., Mariano E. D., Gentile A., Lembke C. G., Zingaretti S. M., Souza G. M. & Menossi M. (2012). A novel stress-induced sugarcane gene confers tolerance to drought, salt and oxidative stress in transgenic tobacco plants. PLoS One. 7:e44697. Camargo M. A. B. & Marenco R. A. (2011). Density, size and distribution of stomata in 35 rainforest tree species in Central Amazonia. Acta Amazonia. 41(2): 205-212. Chaves M. M., Maroco J. P. & Pereira J. S. (2003). Understanding plant responses to drought-from genes to the whole plant. Functional Plant Biology. 30: 239- 264. Farooq M., Hussain M., Wahid A. & Siddique K. H. M. (2012). Drought stress in plants: an overview. In: Aroca R. (Eds.) Plant responses to drought stress. Springer, Berlin/Heidelberg. 1-33. Graça J. P., Rodrigues F. A., Farias J. R. B., Oliveira M. C. N., Hoffmann-Campo C. B. & Zingaretti S. M. (2010). Physiological parameters in sugarcane cultivars submitted to water deficit. Brazilian Journal of Plant Physiology. 22: 189-197. Hoang D. T., Hiroo T. & Yoshinobu K. (2018). Nitrogen use efficiency and drought tolerant ability of various sugarcane varieties under drought stress at early growth stage. Plant Production Science. 22: 250-261. Jaiphong T., Tominaga J., Watanabe K., Nakabaru M., Takaragawa H., Suwa R., Ueno M. & Kawamitsu Y (2016). Effects of duration and combination of drought and flood conditions on leaf photosynthesis, growth and sugar content in sugarcane, Plant Production Science. 19: 427-437. Jangpromma N., Thammasirirak S., Jailsil P. & Songsri P. (2012). Effects of drought and recovery from drought Growth and Physiological Responses of Sugarcane to Drought Stress at an Early Growth Stage 460 Vietnam Journal of Agricultural Sciences stress on above ground and root growth, and water use efficiency in sugarcane (Saccharum officinarum L.). Australian Journal of Crop Science. 6: 1298-1304. Jangpromma N., Songsri P. & Jaisil P. (2010). Rapid assessment of chlorophyll content in sugarcane using a SPAD chlorophyll meter across different water stress conditions. Asian Journal of Plant Science. 9: 368-374. Koonjah S. S., Walker S., Singels A., Van Antwerpen R. & Nayamuth A. R. (2006). A quantitative study of water stress effect on sugarcane photosynthesis. Proceedings of the South African Sugarcane Technologists’ Association. 80: 148-158. Larcher W. (2003). Physiological plant ecology: Ecophysiology and stress physiology of functional groups (4th ed). Springer-Verlag. 401-416. Mafakheri A., Siosemardeh A. & Bahramnejad B. (2010). Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian Journal of Crop Science. 4: 580-585. Maxwell K. & Johnson G. N. (2000). Chlorophyll florescence: a practical guide. Journal of Experimental Botany. 51: 659-668. Meng L., Li L., Chen W., Xu Z. & Liu L. (1999). Effect of water stress on stomatal density, length, width and net photosynthetic rate in rice leaves. Journal of Shenyang Agricultural University. 30: 477-480. Medeiros D. B., Silva E. C., Nogueira R. J. M. C., Teixeira M. M., Buckeridge M. S. (2013). Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theoretical and Experimental Plant Physiology. 25: 213-222. Nonami H. (1998). Plant water relations and control of cell elongation at low water potentials. Journal of Plant Research. 111: 373-382. Robertson M. J., Inman-Bamber N. G., Muchow R. C. & Wood A. W. (1999). Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crop Research. 64: 211-227. Reddy T. Y., Reddy V. R. & Anbumozhi V. (2003). Physiological response of groundnut (Arachis hypogea L.) to drought stress and its amelioration: A critical review. Plant Growth Regulation. 41: 75-88. Silva M. A., Jifon J. L., Santos C. M., Jadoski C. J. & Silva J. A. G. (2013). Photosyntheitc capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Brazilian Archives of Biology and Technology. 56: 735-748. Silva M. A., Jifon J. L., Silva J. A. G. & Sharma V. (2007). Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology. 19: 735-748. Silva M. A., Silva J. A. G., Enciso J., Sharma V. & Jifon J. (2008). Yield components as indicators of drought tolerance of sugarcane. Scientia Agricola. 65: 620-627. Smith D. M., Inman-Bamber N. G. & Thorburn P. J. (2005). Growth and function of the sugarcane root system. Field Crops Research. 92: 169-183. Xu Z. & Zhou Z. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany. 59: 3317- 3325. Yang L., Han M., Zhou G. & Li J. (2007). The changes of water-use efficiency and stoma density of Leymus chinensis along Northeast China Transect. Acta Ecologica Sinica. 27: 16-24.
File đính kèm:
 growth_and_physiological_responses_of_sugarcane_to_drought_s.pdf
growth_and_physiological_responses_of_sugarcane_to_drought_s.pdf

