Green solvent extraction and quality characteristics of passion fruit seed oil (Passiflora edulis Sims var. edulis)
The extraction of oil from passion fruit seeds with acetone, ethanol,
ethyl acetate, isopropanol, and hexane was studied. The effects of the
variables, namely type of solvent, material to solvent ratio,
temperature, and extraction time, were investigated. The highest
extraction yield was 78.52%, which was obtained using ethyl acetate
with a material to solvent ratio of 1/10 at room temperature (28oC)
for 4h using a shaker. This yield was similar to that obtained when
using hexane as a solvent. Our results indicate that ethyl acetate can
replace the conventional hexane solvent in the extraction of oil from
passion fruit seeds. The high content of polyunsaturated fatty acids in
passion fruit seed oil suggests that this product has good potential for
use in the human food, cosmetic, and pharmaceutical industries.

Trang 1

Trang 2
Trang 3

Trang 4

Trang 5

Trang 6
Tóm tắt nội dung tài liệu: Green solvent extraction and quality characteristics of passion fruit seed oil (Passiflora edulis Sims var. edulis)

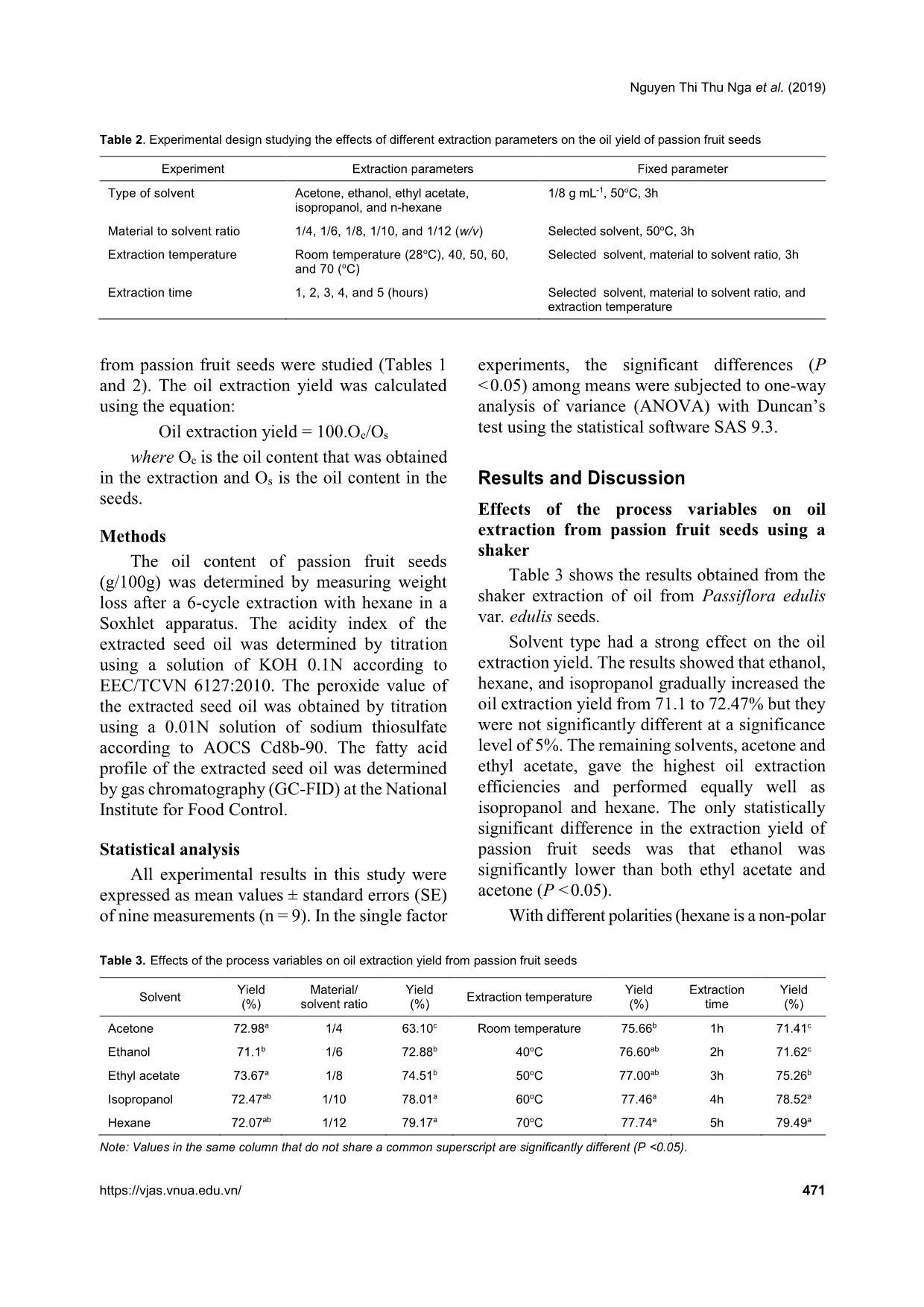
VA) with Duncan’s test using the statistical software SAS 9.3. Results and Discussion Effects of the process variables on oil extraction from passion fruit seeds using a shaker Table 3 shows the results obtained from the shaker extraction of oil from Passiflora edulis var. edulis seeds. Solvent type had a strong effect on the oil extraction yield. The results showed that ethanol, hexane, and isopropanol gradually increased the oil extraction yield from 71.1 to 72.47% but they were not significantly different at a significance level of 5%. The remaining solvents, acetone and ethyl acetate, gave the highest oil extraction efficiencies and performed equally well as isopropanol and hexane. The only statistically significant difference in the extraction yield of passion fruit seeds was that ethanol was significantly lower than both ethyl acetate and acetone (P <0.05). With different polarities (hexane is a non-polar Table 3. Effects of the process variables on oil extraction yield from passion fruit seeds Solvent Yield (%) Material/ solvent ratio Yield (%) Extraction temperature Yield (%) Extraction time Yield (%) Acetone 72.98a 1/4 63.10c Room temperature 75.66b 1h 71.41c Ethanol 71.1b 1/6 72.88b 40oC 76.60ab 2h 71.62c Ethyl acetate 73.67a 1/8 74.51b 50oC 77.00ab 3h 75.26b Isopropanol 72.47ab 1/10 78.01a 60oC 77.46a 4h 78.52a Hexane 72.07ab 1/12 79.17a 70oC 77.74a 5h 79.49a Note: Values in the same column that do not share a common superscript are significantly different (P <0.05). Green Solvent Extraction and Quality Characteristics of Passion Fruit Seed Oil (Passiflora edulis Sims var. edulis) 472 Vietnam Journal of Agricultural Sciences solvent, ethanol and isopropanol are polar solvents, and ethyl acetate and acetone are aprotic polar solvents), different solvents resulted in different extraction efficiencies. The data indicated that the aprotic polar solvents were more suitable for the extraction of oil from purple passion fruit seeds since ethyl acetate and acetone had better oil extraction capacities than ethanol. Oliveira et al. (2014) also found the same result in that acetone was the best solvent compared to ethanol and isopropanol in extracting oil from passion fruit seeds. In accordance with Directive 2009/32/EC of the European Union on April 23, 2009, both ethyl acetate and acetone are allowed to be used as solvents in the food industry because they generate less waste than other solvents. Because the boiling point of ethyl acetate is 77oC, higher than that of acetone (56oC), ethyl acetate was selected for the next experiment due to less solvent loss during oil recovery. The oil content in the extracts strongly depended on the material to solvent ratio. There was an increase of the passion fruit seed oil yield with an increase of the material to solvent ratio (w/v). The material to solvent ratio of 1:12 (w/v) showed the highest amount of oil but was not significantly different from the ratio of 1:10 (w/v) (P >0.05). The opposite behavior is reported by Oliveira et al. (2014) who found that the ratio 1:4 of passion fruit seeds to solvent was the best ratio to extract oil from these seeds. Maybe differences between yellow and purple passion fruit seeds led to the differences in findings between the results of Oliveira et al. (2014) and our study. A high material to solvent ratio could promote an increased concentration gradient, producing a higher chance of oil coming into contact with the extraction solvent, resulting in an increased diffusion rate that allows for greater extraction of materials by the solvent (Cacace & Mazza, 2003). These results were consistent with the mass transfer principle. However, the active component yields would not continue to increase once equilibrium had been reached (Herodež et al., 2003). An equilibrium constant trend was observed at the material to solvent ratio of 1:10 (w/v) that indicated a sufficient amount of extracting solvent was used in the extraction of oil from passion fruit seeds and thus, this ratio was chosen for the determination of the extraction temperature and extraction time. Regarding the extraction temperature, the results indicated a significant increase in the oil extraction yield when the temperature increased from room temperature (28oC) to 60oC (P <0.05) but the oil extraction efficiency did not significantly differ between the temperatures of 60 and 70oC (P >0.05). When performing extractions at low and mediate temperatures, from room temperature to 50oC, the flexibility of the fatty constituents in the materials was not strongly promoted; hence, the extraction efficiency was around 76%. When the temperature was raised to 60oC, the thermal conductivity increased, which made the diffusion coefficient of the solutes increase, consequently increasing their solubility (Al-Farsi & Chang, 2007) and thus, accelerating the whole extraction up to 78%. Although increasing the temperature to 60oC resulted in a 2% increase in the oil extraction yield, 60oC is not only closer to the boiling point of ethyl acetate (77oC), which might cause significant losses in the solvent, energy consumption, and economic efficiency, but small constituents of materials are also easily dissolved at this temperature, leading to difficulties in oil recovery. Therefore, room temperature (28oC) was selected for the extraction of oil from passion fruit seeds in the subsequent step. The evaluation of the extraction time showed that the extraction capacity of passion fruit seeds increased sharply when the extraction time was increased to 4h, but at longer extraction times, the efficiency did not increase significantly (P >0.05). This finding is in agreement with the report of Oliveira et al. (2014) who reported that the highest oil extraction capacity value from passion fruit seeds was obtained after 4h of extraction. These phenomena could be explained by Fick’s second law of diffusion, predicting that a final equilibrium between the solute concentration in the plant matrix and in the solvent might be reached after a certain time. Nguyen Thi Thu Nga et al. (2019) https://vjas.vnua.edu.vn/ 473 Chemical and quality characteristics of Passiflora edulis var. edulis seed oil According to the chemical characteristics of passion fruit seed oil (Table 4), the oil content of Passiflora edulis var. edulis seeds was 27.28% on a dry basis indicating that these seeds were a good oil source when compared to soybean seeds, which contain about 25% on a dry basis (Hammad et al., 2012). The oil content reported in this study was higher than the values of 18.5% for P. edulis var. edulis seeds and 20.6% for P. edulis var. flavicarpa seeds in Uganda found by Nyanzi et al. (2005), and lower than the figures of 30.39% and 30.22% for yellow passion fruit seeds in Brazil found by Malacrida & Jorge (2012) and Silva et al. (2015), respectively. Differences in the oil contents of P. edulis could be due to genetic, climate, and geographical attributes. The acidity index of the extracted oil provides important information on the condition of oil conservation. A high acidity index indicates that the oil was partly oxidized or degraded. The value found in this study (1.61 ± 0.05 mg KOHg-1) was equal with the value found by Silva et al. (2015) and 1.5 times lower than the quality parameter of 4.0 mg KOHg-1 according to The Codex Alimentarius Commission (2008). This result indicates that the studied oil can be used for food purposes as it meets the required standard. The determination of the peroxide value is used as an indicator of lipid oxidation. High peroxide values of extracted oil indicate that the oil was exposed to oxidative processes during the preparation of the raw material, extraction, or oil storage. In our study, the peroxide value of the seed oil from P. edulis var. edulis was 2.5 times lower than peroxide value of the seed oil from P. edulis var. flavicarpa found by Silva et al. (2015) of 1.54 ± 0.14 meqO2kg -1 and far lower than the value that the Codex Alimentarius Commission established for refined and crude oils in 2008 at 15 meqO2kg -1. The GC analysis of the fatty acids of the P. edulis var. edulis seed oil showed a high content of unsaturated fatty acids such as linoleic and oleic (Table 5). Linoleic acid (C18:2n6), an essential polyunsaturated omega-6 fatty acid for humans, was the most dominant fatty acid making up 75.7% of the total fatty acids and was followed by oleic acid (C18:1n9) at 9%. These data were in agreement with the results reported by Nyanzi et al. (2005) in that linoleic and oleic acids made up the majority of the fatty acids found in the extracted oil of P. edulis var. edulis seeds with the values of 74.3% for linoleic acid and 13.6% for oleic acid. In comparison to the fatty acid compositions of soybean oil and sunflower oil, two popular vegetable oils used in the food, cosmetic, and pharmaceutical industries (Rabasco & Gonzalez, 2000), the percentage of saturated fatty acids in Table 4. Chemical characteristics of P. edulis var. edulis seeds oil Component Value* Oil content (%) 27.28 ± 0.54 Acidity index (mg KOH g-1) 1.61 ± 0.05 Peroxide value (meqO2 kg -1) 0.62 ± 0.03 Note: * Each value is the mean ± SD of triplicate extractions and determinations. Table 5. Fatty acid composition of P. edulis var. edulis seed oil Fatty acids Composition (%) Oleic acid (C18:1n9c) 9 Linoleic acid (C18:2n6) 75.7 Docosahexaenoic acid (C22:6n3) 0.94 Saturated fatty acid (SFA) 14 Unsaturated fatty acid (UFA) 86 Green Solvent Extraction and Quality Characteristics of Passion Fruit Seed Oil (Passiflora edulis Sims var. edulis) 474 Vietnam Journal of Agricultural Sciences passion fruit seed oil amounted to 14%, while the value obtained for soybean oil was slightly higher (15.1%) and the value for sunflower oil was a little lower (12.36%). Among the monounsaturated fatty acids, oleic acid was the main representative and its content was the lowest in passion fruit seed oil (9%) and highest in soybean oil (21.73%), while the level of oleic in sunflower oil was 15.93% (Zambiazi et al., 2007). Linoleic was the dominant fatty acid among the polyunsaturated fatty acids (PUFAs) and the linoleic acid amount in passion fruit seed oil was higher than the vales determined in sunflower oil and soybean oil, which were 71% and 56%, respectively. It can also be noted that sunflower oil’s high PUFA content makes this oil a good salad oil source (Sullivan, 1980), and with the primary constituents of oleic, linoleic, and linolenic acids, soybean oil and sunflower oil can be employed in cosmetic products and pharmaceutical formulations (Rabasco & Gonzalez, 2000). Hence, the high content of PUFAs of the studied oil indicates this product is a good source of essential PUFAs for human use. Conclusions In this study, the effects of process variables on the solvent extraction of oil from passion fruit seeds were investigated using a shaker. The highest extraction yield was 78.52%, which was obtained using ethyl acetate at the ratio of 1:10 material to solvent at room temperature (28oC) for 4h, and was similar to the results obtained with the conventional solvent hexane. Our results indicate that passion fruit seed oil extracted by ethyl acetate has a high content of PUFAs (around 77%) and thus, has good potential for use in the human food, cosmetic, and pharmaceutical industries. Acknowledgements The authors would like to acknowledge Vietnam National University of Agriculture for providing financial support under the Research Grant Scheme (Grant agreement T2017-08-55). References Al-Farsi M. A. & Chang Y. L. (2007). Optimization of phenolics and dietary fibre extraction from date seeds. Food Chemistry. 108(3): 977-985. Cacace J. E. & Mazza G. (2003). Mass transfer process during extraction of phenolic compounds from milled berries. Journal of Food Engineering. 59: 379-389. Codex Alimentarius. Fats, oils and related products (revised 2001). Second edition. 8. Cowan M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews. 12(4): 564-582. Directive 2009/32/EC (2009). The European Parliament and the Council of the European Union. on extraction solvents used in the production of foodstuffs and food ingredients. Official Journal of the European Union. 141: 3-11. Hammad K. H. A., Zhmurko V. V. & Avksentyeva O. A. (2012). Seed Protein and Oil Content of the Soybean Cultivars under Different Climate Condition (Glycine max (L.) Merr.). American-Eurasian Journal of Agricultural and Enviromental Science. 12(5): 603-607. Herodež Š. S., Hadolin M., Škerget M. & Knez Ž. (2003). Solvent extraction study of antioxidants from Melissa officinalis L. leaves. Food Chemistry. 80: 275-282. Malacrida C. R. & Jorge N. (2012). Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Brazilian Archives of Biology and Technology. 55(1): 127-134. Nyanzi S. A., Carstensen B. & Schwack W. A. (2005). A Comparative Study of Fatty Acid Profiles of Passiflora Seed Oils from Uganda. Journal of American Oil Chemists’ Society. 82(1): 41-44. Oliveira R. C., Barros S. T. D. & Gimenes M. L. (2013). The extraction of passion fruit oil with green solvents. Journal of Food Engineering 117: 458-463. Oliveira R. C., Guedes T. A., Gimenes M. L. & Barros S. T. D. (2014). Effect of process variables on the oil extraction from passion fruit seeds by conventional and non-conventional techniques, Acta Scientiarum Technol. 36: 87-91. Rabasco A. A. M. & González R. M. L. (2000). Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites. 51: 74-96. Rehm S. & Espig G. (1984). The Cultivated Plants of the Tropics and Subtropics, Eugen Ulhmer Publishers, Stuttgart, Germany. 185-186 (in German). Silva R. M., Placido G. R., Silva M. A. P., Castro C. F. S., Lima M. S. & Caliari M. (2015). Chemical characterization of passion fruit (Passiflora edulis f. flavicarpa) seeds, African Journal of Biotechnology. 14: 1230-1233. Sullivan F. E. (1980). Sunflower oil processing from crude to salad oil, Journal of the American Oil Chemists' Society. 57: 845-847. Zambiazi R. C., Przybylski R., Zambiazi M. W. & Mendonça C. B. (2007). Fatty acid composition of vegetable oils and fats. Boletim Centro de Pesquisa de Processamento de Alimentos. 25: 111-120.
File đính kèm:
 green_solvent_extraction_and_quality_characteristics_of_pass.pdf
green_solvent_extraction_and_quality_characteristics_of_pass.pdf

