Dietary supplementation with sesame seeds to improve semen quality of Ho cocks
High levels of polyunsaturated fatty acids in chicken spermatozoa make them susceptible to lipoperoxidation and reduce their fertility. This study was conducted to assess the effect of sesame seed suplementation in the diet on the semen quality of Ho cocks. Eighteen 13-14 month-old cocks were randomly divided into three groups and were assigned to one of the following treatments: 0% SS (control), 5% SS, or 7% sesame seeds per kg of diet for ten consecutive weeks after a two-week adaptation period. Semen characteristics were evaluated once a week. In the 7% sesame seed treatment group, seminal traits including semen ejaculate volume (1.02mL), sperm concentration (3.68 x 109 sperm), and abnormal spermatozoa (10.51% were improved (P <0.05) compared="" to="" the="" control="" group="" (0.82ml,="" 2.81="" x="" 109="" sperm,="" and="" 11.04%="" for="" semen="" ejaculate="" volume,="" sperm="" concentration,="" and="" abnormal="" spermatozoa,="" respectively).="" supplementation="" with="" sesame="" seeds="" did="" not="" significantly="" affect="" sperm="" motility,="" mass="" movement,="" or="" semen="" ph.="" our="" results="" demonstrate="" that="" sesame="" seed="" supplementation="" at="" 7%="" successfully="" improved="" the="" ejaculate="" volume,="" sperm="" concentration,="" and="" normal="" spermatozoa="" percentage="" of="" ho="">

Trang 1

Trang 2
Trang 3

Trang 4
Trang 5

Trang 6
Trang 7
Trang 8
Trang 9

Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Dietary supplementation with sesame seeds to improve semen quality of Ho cocks

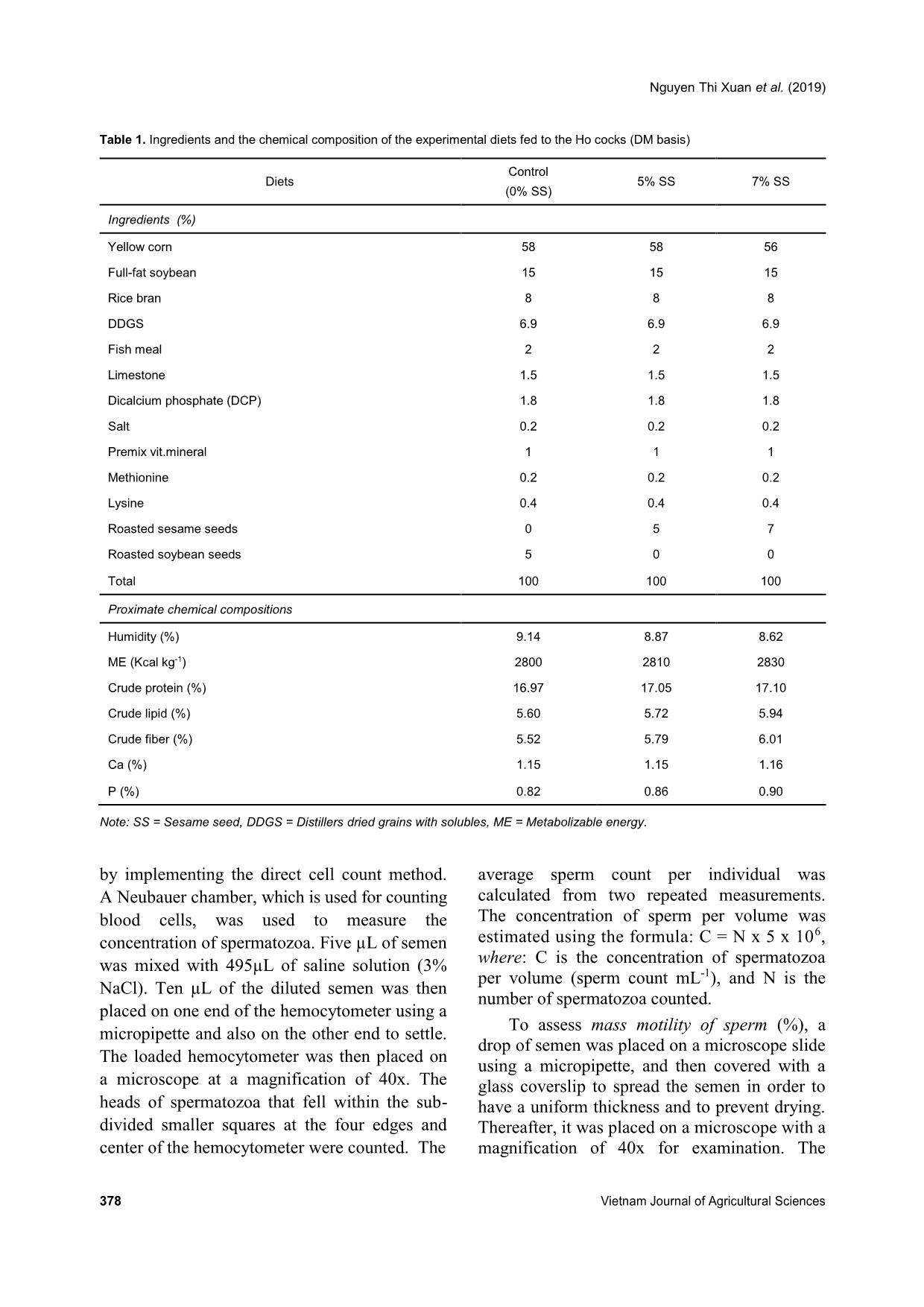
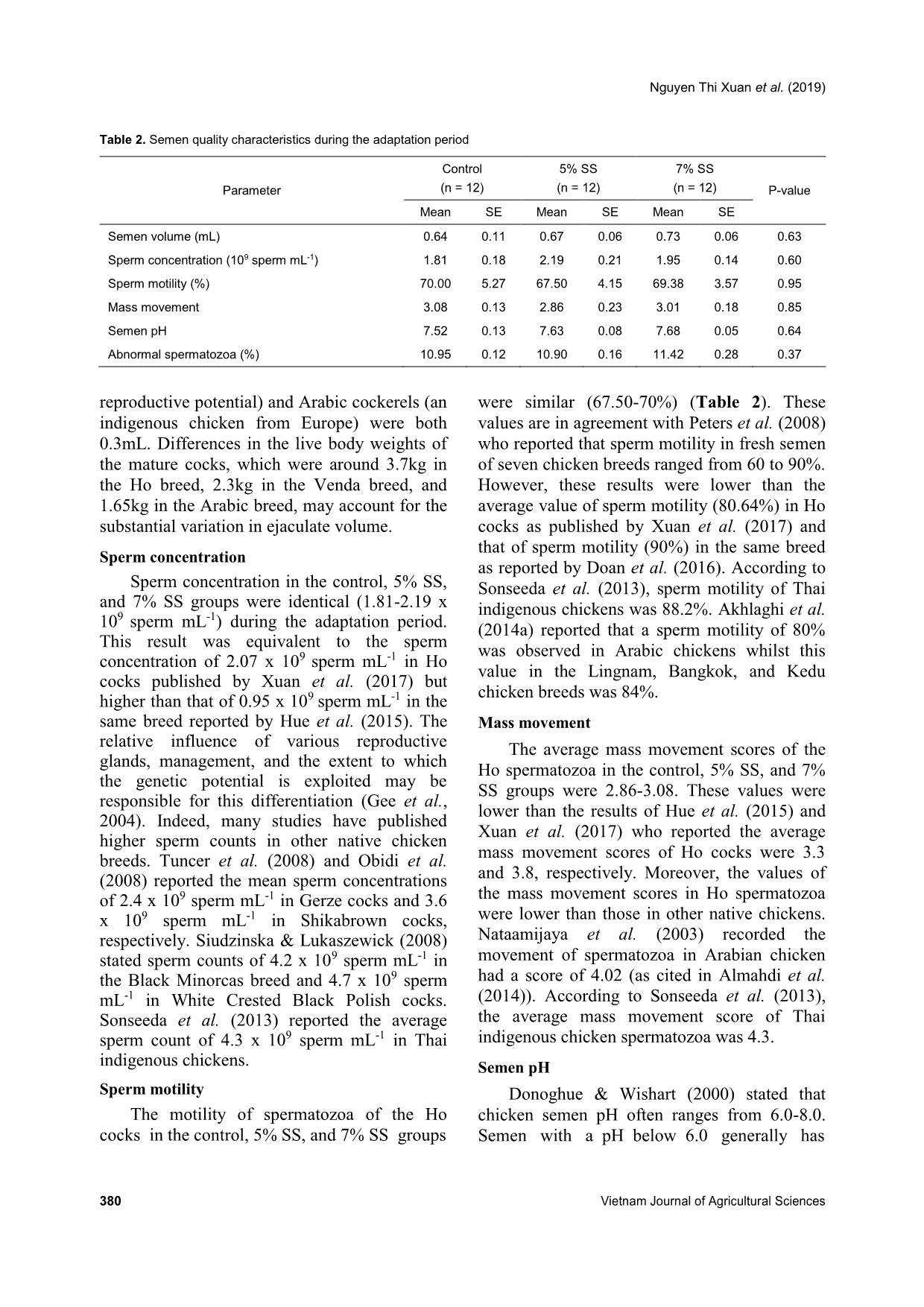
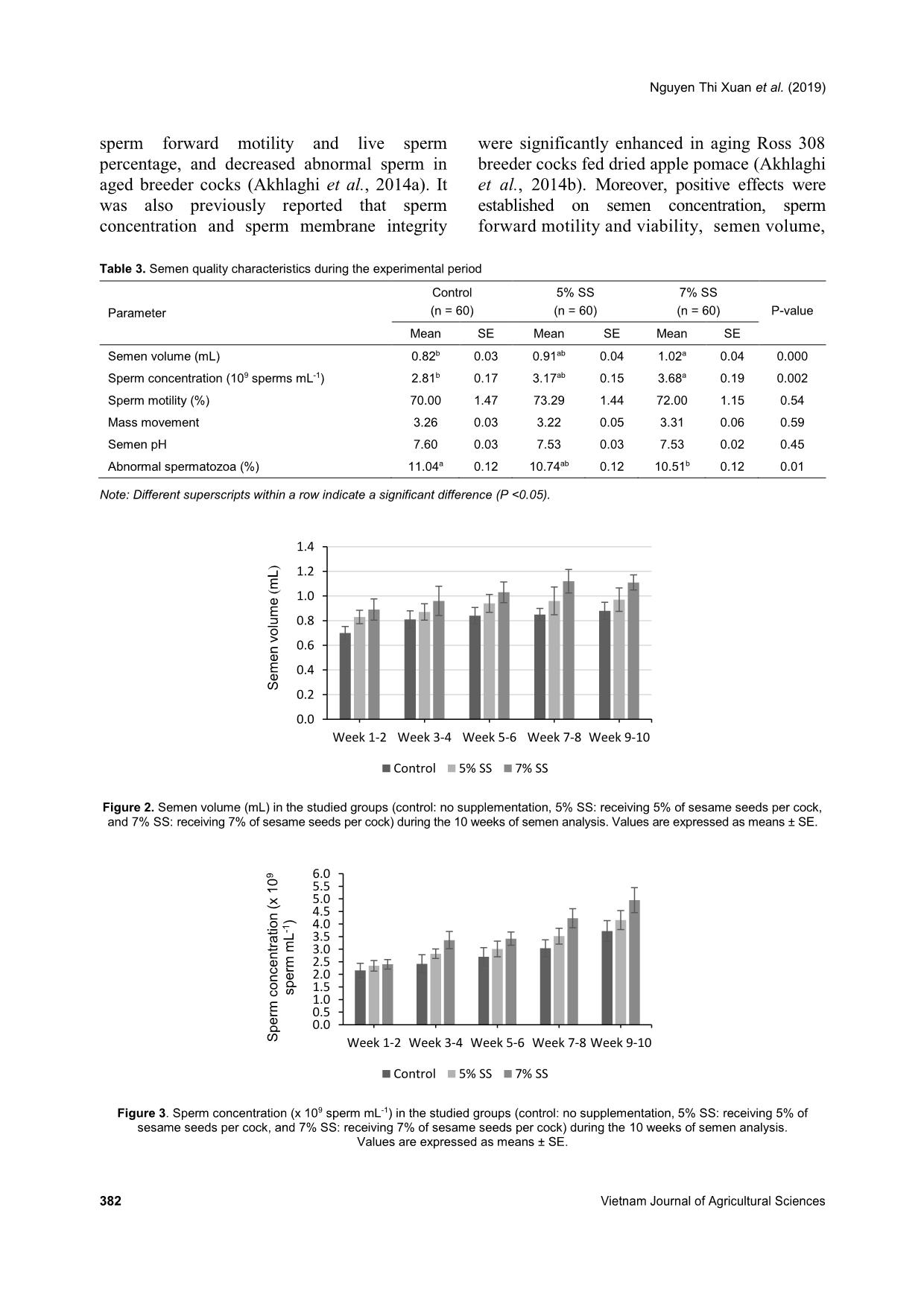
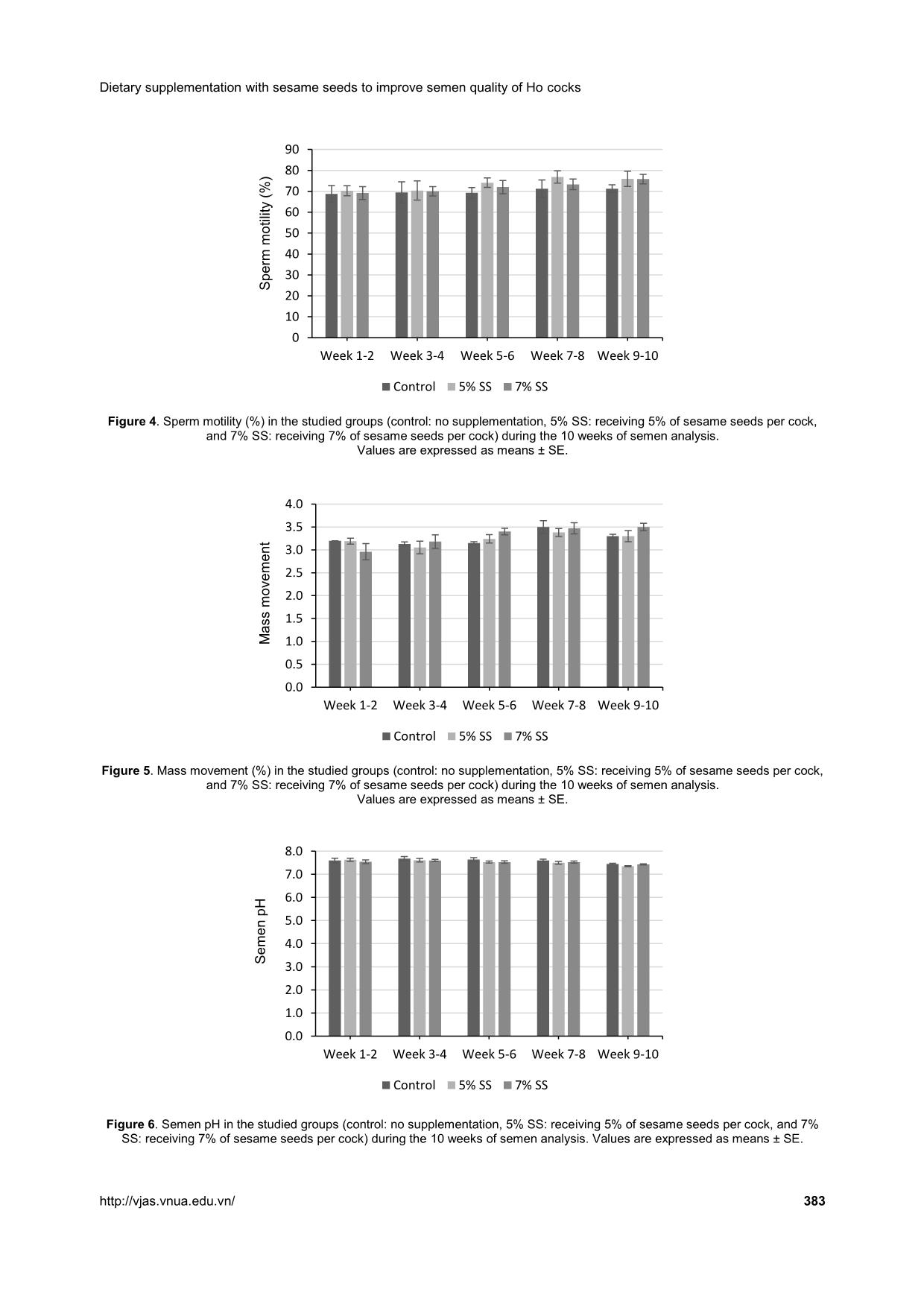
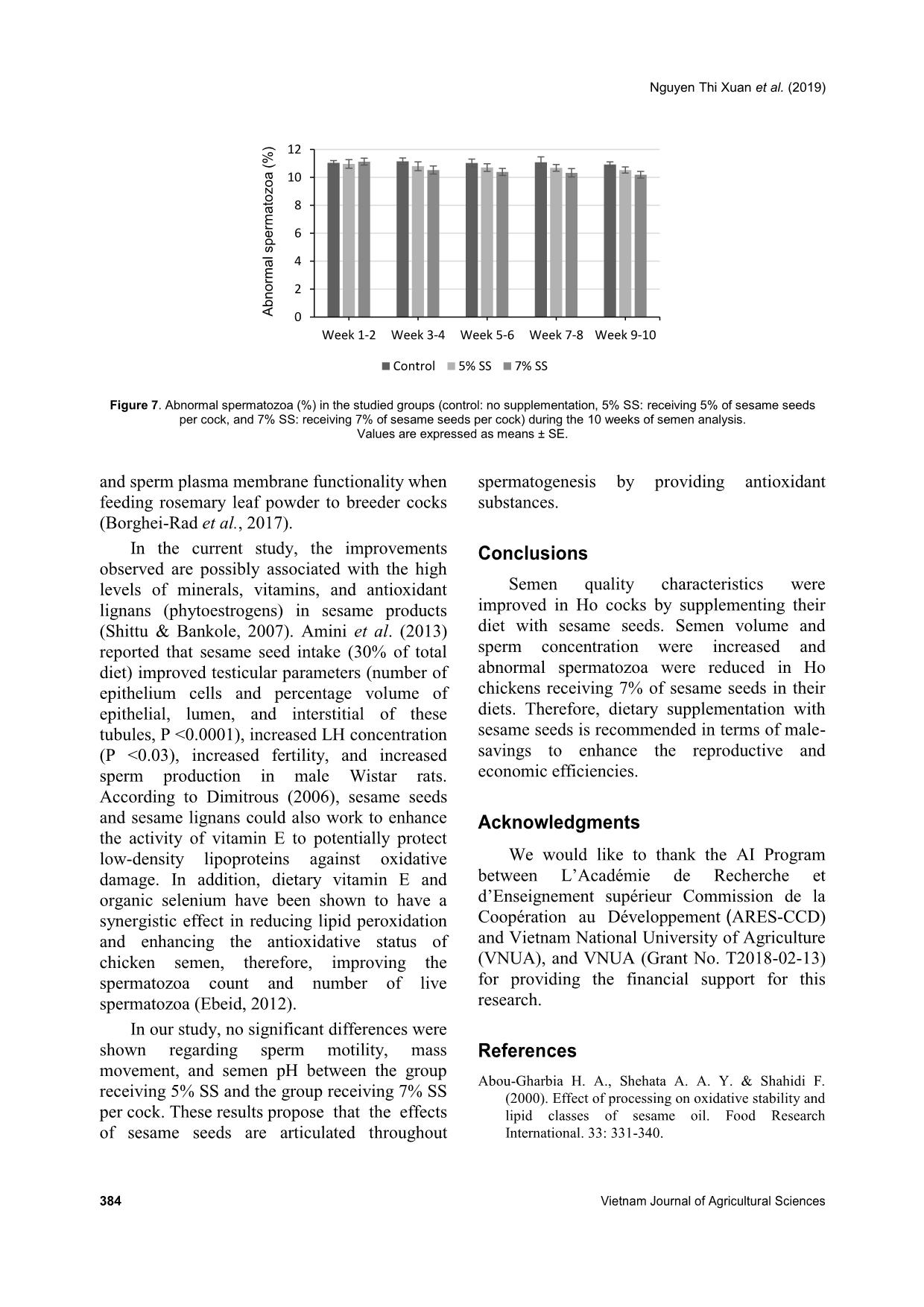
group from weeks 1-10 (Figures 5 and 6, respectively). Concerning the abnormal spermatozoa trait, significant improvement in the group fed 7% SS was observed compared to the control (Figure 7). Chicken spermatozoa are rich in PUFAs which make them vulnerable to oxidative stress and lipid peroxidation (Surai et al., 1998; Eid et al., 2006) and therefore, reduce their motility and fertility (Sanocka & Kurpisz, 2004; Khan, 2011). To lessen chicken spermatozoa quality loss, different antioxidants have been investigated. Lycopene, a carotenoid existing in vegetables and fruits, showed a positive effect on semen volume and sperm concentration of broiler breeder males when supplemented in drinking water (Mangiagalli et al., 2010). Likewise, dietary ginger powder improved Nguyen Thi Xuan et al. (2019) 382 Vietnam Journal of Agricultural Sciences sperm forward motility and live sperm percentage, and decreased abnormal sperm in aged breeder cocks (Akhlaghi et al., 2014a). It was also previously reported that sperm concentration and sperm membrane integrity were significantly enhanced in aging Ross 308 breeder cocks fed dried apple pomace (Akhlaghi et al., 2014b). Moreover, positive effects were established on semen concentration, sperm forward motility and viability, semen volume, Table 3. Semen quality characteristics during the experimental period Parameter Control (n = 60) 5% SS (n = 60) 7% SS (n = 60) P-value Mean SE Mean SE Mean SE Semen volume (mL) 0.82b 0.03 0.91ab 0.04 1.02a 0.04 0.000 Sperm concentration (109 sperms mL-1) 2.81b 0.17 3.17ab 0.15 3.68a 0.19 0.002 Sperm motility (%) 70.00 1.47 73.29 1.44 72.00 1.15 0.54 Mass movement 3.26 0.03 3.22 0.05 3.31 0.06 0.59 Semen pH 7.60 0.03 7.53 0.03 7.53 0.02 0.45 Abnormal spermatozoa (%) 11.04a 0.12 10.74ab 0.12 10.51b 0.12 0.01 Note: Different superscripts within a row indicate a significant difference (P <0.05). Figure 2. Semen volume (mL) in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. Figure 3. Sperm concentration (x 109 sperm mL-1) in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10 S e m e n v o lu m e (m L ) Control 5% SS 7% SS 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10S p e rm c o n c e n tr a ti o n ( x 1 0 9 s p e rm m L -1 ) Control 5% SS 7% SS Dietary supplementation with sesame seeds to improve semen quality of Ho cocks 383 Figure 4. Sperm motility (%) in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. Figure 5. Mass movement (%) in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. Figure 6. Semen pH in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. 0 10 20 30 40 50 60 70 80 90 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10 S p e rm m o ti lit y ( % ) Control 5% SS 7% SS 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10 M a s s m o v e m e n t Control 5% SS 7% SS 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10 S e m e n p H Control 5% SS 7% SS Nguyen Thi Xuan et al. (2019) 384 Vietnam Journal of Agricultural Sciences Figure 7. Abnormal spermatozoa (%) in the studied groups (control: no supplementation, 5% SS: receiving 5% of sesame seeds per cock, and 7% SS: receiving 7% of sesame seeds per cock) during the 10 weeks of semen analysis. Values are expressed as means ± SE. and sperm plasma membrane functionality when feeding rosemary leaf powder to breeder cocks (Borghei-Rad et al., 2017). In the current study, the improvements observed are possibly associated with the high levels of minerals, vitamins, and antioxidant lignans (phytoestrogens) in sesame products (Shittu & Bankole, 2007). Amini et al. (2013) reported that sesame seed intake (30% of total diet) improved testicular parameters (number of epithelium cells and percentage volume of epithelial, lumen, and interstitial of these tubules, P <0.0001), increased LH concentration (P <0.03), increased fertility, and increased sperm production in male Wistar rats. According to Dimitrous (2006), sesame seeds and sesame lignans could also work to enhance the activity of vitamin E to potentially protect low-density lipoproteins against oxidative damage. In addition, dietary vitamin E and organic selenium have been shown to have a synergistic effect in reducing lipid peroxidation and enhancing the antioxidative status of chicken semen, therefore, improving the spermatozoa count and number of live spermatozoa (Ebeid, 2012). In our study, no significant differences were shown regarding sperm motility, mass movement, and semen pH between the group receiving 5% SS and the group receiving 7% SS per cock. These results propose that the effects of sesame seeds are articulated throughout spermatogenesis by providing antioxidant substances. Conclusions Semen quality characteristics were improved in Ho cocks by supplementing their diet with sesame seeds. Semen volume and sperm concentration were increased and abnormal spermatozoa were reduced in Ho chickens receiving 7% of sesame seeds in their diets. Therefore, dietary supplementation with sesame seeds is recommended in terms of male- savings to enhance the reproductive and economic efficiencies. Acknowledgments We would like to thank the AI Program between L’Académie de Recherche et d’Enseignement supérieur Commission de la Coopération au Développement (ARES-CCD) and Vietnam National University of Agriculture (VNUA), and VNUA (Grant No. T2018-02-13) for providing the financial support for this research. References Abou-Gharbia H. A., Shehata A. A. Y. & Shahidi F. (2000). Effect of processing on oxidative stability and lipid classes of sesame oil. Food Research International. 33: 331-340. 0 2 4 6 8 10 12 Week 1-2 Week 3-4 Week 5-6 Week 7-8 Week 9-10 A b n o rm a l s p e rm a to z o a ( % ) Control 5% SS 7% SS Dietary supplementation with sesame seeds to improve semen quality of Ho cocks 385 Abu M. M. T., Mohammad M. U. B., Raihana N. F., Nasrin S. J. & Md. Bazlur B. R. M. (2013). Evaluation of semen quality among four chicken lines. IOSR Journal of Agriculture and Veterinary Science. 6: 7-13. Akhlaghi A., Ahangari Y. J., Navidshad B., Pirsaraei Z. A., Zhandi M., Deldar M. H., Rezvani R., Dadpasand M., Hashemi S. R., Poureslami R. & Peebles E. D. (2014a). Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb 500 breeder roosters fed diets containing dried ginger rhizomes (Zingiber officinale). Poultry Science. 93: 1236-1243. Akhlaghi A., Jafari Ahangari Y., Zhandi M. & Peebles E. D. (2014b). Reproductive performance, semen quality, and fatty acid profile of spermatozoa in senescent broiler breeder roosters as enhanced by the long-term feeding of dried apple pomace. Animal Reproduction Science. 147: 64-73. Almahdi A. B., Ondho Y. S. & Sutopo D. (2014). Comparative Studies of Semen Quality on Different Breed of Chicken in Poultry Breeding Center Temanggung-Central Java. International Refereed Journal of Engineering and Science (IRJES). 3(2): 94- 103. Amini M. J., Hassani B. H., Nikzad H., Taherian A. & Salehi M. (2013). Effect of Diet Contains Sesame Seed on Adult Wistar Rat Testis. International Journal of Morphology. 31: 197-202. Ax R. L., Dally M., Didion B. A. & Lenz R. W. (2000). Semen evaluation. In: Hafez B. & Hafez E. S. E. Reproduction in Farm Animals (7th ed.). Philadelphia: Lippincott Williams & Wilkins. DOI: 10.1002/9781119265306.ch25. Bah G. S., Chaudhari S. U. R. & Al-Amin J. D. (2001). Semen Characteristics of Local Breeder Cocks in the Sahel Region of Nigeria. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux. 54(2): 153-158. Biswas A., Mohan J. & Sastry K. V. H. (2009). Effect of higher dietary vitamin E concentrations on physical and biochemical characteristics of semen in Kadaknath cockerels. British Poultry Science. 50: 733-738. Borghei-Rad S. M., Zeinoaldini S., Zhandi M., Moravej H. & Ansari M. (2017). Feeding rosemary leaves powder ameliorates rooster age-related subfertility. Theriogenology. 101: 35-43. Dimitrous B. (2006). Sources of natural phenolic antioxidants. Trends in Food Science and Technology. 17: 505-512. Doan B. H., Tuan H. A., Hang D. L. & Thinh N. H. (2016). Effects of artificial insemination methods on reproductive performance of Ho breed of chicken. Vietnam Journal of Agricultural Sciences. 14: 727- 733 (in Vietnamese with English abstract). Donoghue A. M. & Wishart G. J. (2000). Storage of poultry semen. Animal Reproduction Science. 62: 213-232. Duy N. V., Moula N., Luc D. D., Dang P. K., Hiep D. T., Doan B. H., Ton V. D. & Farnir F. (2015). Ho Chicken in Bac Ninh Province (Vietnam): From an Indigenous Chicken to Local Poultry Breed. International Journal of Poultry Science. 14(9): 521- 528. Ebeid T. A. (2012). Vitamin E and organic selenium enhances the antioxidative status and quality of chicken semen under high ambient temperature. British Poultry Science. 53: 708-714. Eid Y., Ebeid T. & Younis H. (2006). Vitamin E supplementation reduces dexamethasone-induced oxidative stress in chicken semen. British Poultry Science. 47: 350-356. Gee G. F., Bertschinger H., Donoghue A. M., Blanco J. & Soley J. (2004). Reproduction in Nondomestic Birds: Physiology, Semen Collection, Artificial Insemination and Cryopreservation. Avian and Poultry Biology Reviews. 15: 47-101. Gharby S., Harhar H., Bouzoubaa Z., Asdadi A., El Yadini A. & Charrouf Z. (2017). Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. Journal of the Saudi Society of Agricultural Sciences. 16: 105-111. Hue D. T., Luc D. D., Dung N. T., Thinh N. H. & Ton V. D. (2015). Semen quality of Ho chicken and some affected factors. Conference of Sustainable Livestock Development Hanoi: Agricultural University Press. 1- 7 (in Vietnamese with English abstract). Jafari A. Y., Parizadian B. & Zamani M. (2013). The impact of organic selenium supplementation on rooster semen quality in liquid condition. Poultry Science Journal. 1(1): 23-31. Khan R. U. (2011). Antioxidants and poultry semen quality. World's Poultry Science Journal. 67: 297- 308. Lake P. (1962). Artificial Insemination in poultry. In: Maile J. P. e. (Ed.). The semen of animals and artificial insemination. Bucks, England: Commonwealth Agriculture Bureau. Mangiagalli M. G., Martino P. A., Smajlovic T., Guidobono Cavalchini L. & Marelli S. P. (2010). Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. British Poultry Science. 51: 152-157. Masindi L. & Mphaphathi M. M. (2016). The characterisation and cryopreservation of Venda chicken semen. Asian Pacific Journal of Reproduction. 5(2): 132-139. Min Y., Sun T., Niu Z. & Liu F. (2016). Vitamin C and Nguyen Thi Xuan et al. (2019) 386 Vietnam Journal of Agricultural Sciences vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Animal Reproduction Science. 171: 1-6. Obidi J. A., Onyeanusi B. I., Rekwot P. I., Ayo J. O. & Dzenda T. (2008). Seasonal variations in seminal characteristics of characteristics of Shikabrown breeder cocks. International Journal of Poultry Science. 7: 1219-1223. Pathak N., Rai A. K., Kumari R. & Bhat K. V. (2014). Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacognosy reviews. 8(16): 147-155. Peters S. O., Shoyebo O. D., Ilori B. M., Ozoje M. O., Ikeobi C. O. N. & Adebambo O. A. (2008). Semen quality traits of seven strain of chickens raised in the humid tropics. International Journal of Poultry Science. 7: 949-953. Sanocka D. & Kurpisz M. (2004). Reactive oxygen species and sperm cells. Reproductive Biology and Endocrinology. 2: 12. Shittu R. K. & Bankole M. N. (2007). Sesame leaves intake improve and increase epididymal spermatocytes reserve in adult male Sprague Dawley rat. Scientific research and essays. 2: 319-324. Siudzinska A. & Lukaszewick E. (2008). The effect of breed on freezability of semen of fancy fowl. Animal Science Papers and Reports. 4: 331-340. Sonseeda P., Vongpralub T. & Laopaiboon B. (2013). Effects of Environmental Factors, Ages and Breeds on Semen Characteristics in Thai Indigenous Chickens: A One-year Study. Thai Journal of Veterinary Medicine. 43(3): 347-352. Surai P. F., Cerolini S., Wishart G. J., Speake B. K., Noble R. C. & Sparks N. H. (1998). Lipid and antioxidant composition of chicken semen and its susceptibility to peroxidation. Poultry and Avian Biology Reviews. 9: 11-23. Tha D. D. (2006). Artificial insemination technology for animals. Labor Social Publishing House, Hanoi (in Vietnamese). Tham L. T., Thu D. V., Binh D. V., Khoi T. X., Hue L. T., Thai N. X. & Binh D. V. (2017). Assessment of semen quality and artificial insemination for Dong Tao chicken. Vietnam Journal of Agricultural Sciences. 15(6): 755-763 (in Vietnamese with English abstract). Tuncer P. B., Kinet H. & Ozdogan N. (2008). Evaluation of some spermatological characteristics in Gerze cocks. Ank Univ Vet Derg. 55: 99-102. Xuan N. T., Duy N. V., Hue D. T., Luc D. D. & Ton V. D. (2017). Fertilizing ability of Ho cock semen by different insemination doses and dilution rates. International Conference on: Animal production in Southeast Asia current status and future. Agricultural University Press, Hanoi, Vietnam. 48-57.
File đính kèm:
 dietary_supplementation_with_sesame_seeds_to_improve_semen_q.pdf
dietary_supplementation_with_sesame_seeds_to_improve_semen_q.pdf

